35 lewis dot diagram for o2
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ... A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Diatomic Oxygen).Note that Diatomic Oxygen is often called Molecular Oxygen or just Oxy...
Silicon dioxide (SiO2) lewis structure. As you see in the above SiO2 lewis dot structure, we convert 2 lone pairs of electrons of each oxygen atom to a covalent bond. So, both atoms (silicon and oxygen) have 8 electrons in their valence shell. Hence we got our best and stable Silicon dioxide lewis structure.
Lewis dot diagram for o2
Lewis diagram for the ether c2h5oc2h5 17.11.2021 · Molecular orbital diagram practice worksheet A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...
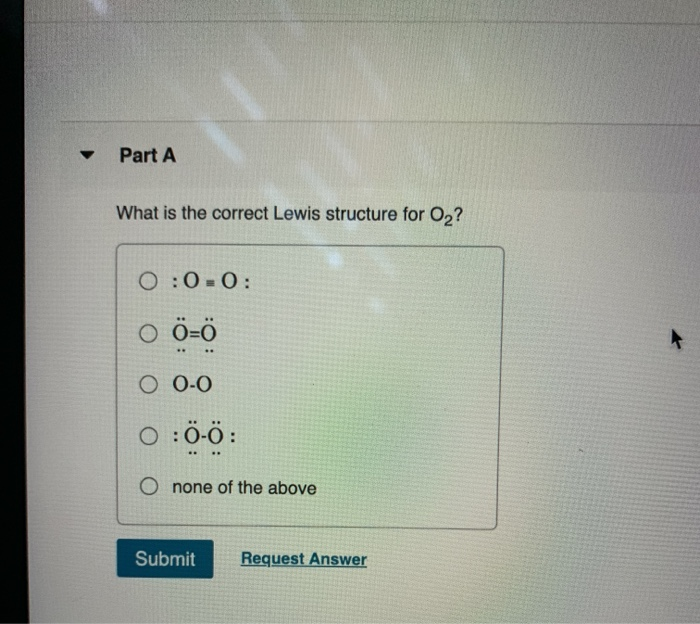
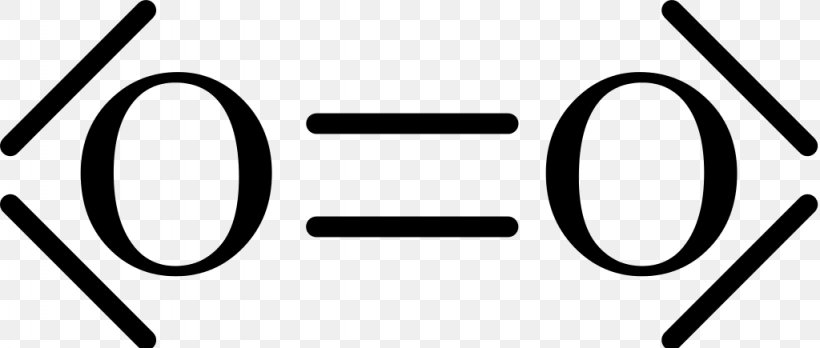
Lewis dot diagram for o2. Feb 20, 2019 · O2 is an allotrope of oxygen and is made out of two oxygen atoms bound together. Although the chemical formula for this allotrope is O2, it is frequently just referred to as oxygen. O2 or dioxygen’s particular formulation is one of the most common elemental compounds on the planet, constituting around 20.8% of the Earth’s atmosphere. Live. •. See the Big List of Lewis Structures. Transcript: OK, we're going to do the Lewis dot structure for O2. Let's start. Looking on the periodic table, we can find Oxygen in group 6 or 16, and that means it has 6 valence electrons. But we have two of them so we'll multiply that by 2. That gives us a total of 12 valence electrons. Cl2 Br2 H2 O2 N2 HCl DOUBLE bond atoms that share two e- pairs (4 e-) O O TRIPLE bond atoms that share three e- pairs (6 e-) N N Draw Lewis Dot Structures You may represent valence electrons from different atoms with the following symbols x, , CO2 NH3 Draw the Lewis Dot Diagram for polyatomic ions Count all valence e- needed for covalent bonding Add or subtract other electrons based on the ... Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a way that's easy for you to ...
Nov 30, 2018 · Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or Oct 18, 2021 · Worksheets are Lewis dot structures and molecule geometries work 5 11a electron diagrams and lewis structures wkst key Practice problems h s so ch br hcn Lewis structures shapes and polarity Work 13 Ws lewis structures covalent Lewis. 22.11.2021 · The Lewis structure, also called as electron dot structure, ... The molecular orbital diagram is a diagrammatic representation of showing how chemical bonding is taking place within a molecule. ... Previous Article O2 Lewis Structure, Molecular Geometry, and Hybridization. In the Lewis-dot structure the valance electrons are shown by 'dot'. The given molecule is, As we know that rubidium has '1' valence electrons, iodine has '7' valence electrons and oxygen has '6' valence electrons.
Steps to draw the lewis dot structure for CN¯ ... By following the same steps of the CN – lewis structure, you can also make a lewis diagram for other cyanide ions. 1. lewis structure for CN. ∴ As there is only 9 valence electron present in CN molecule. ... O2, B2, etc. ⇒ A hetero-nuclear ... 27.11.2021 · The Lewis structure is a dot diagram to determine how many lone valence electrons are present and absent within an atom. Moreover, it is easy to figure out which bond has been formed between the atoms of a molecule, with the help of this diagram. The O2 molecule forms a double covalent bond between two shared pairs of electrons. A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t... 17.11.2021 · Molecular orbital diagram practice worksheet
Lewis diagram for the ether c2h5oc2h5






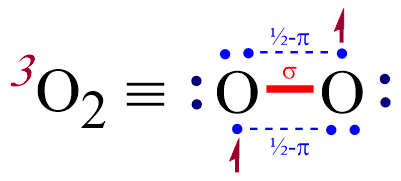
















0 Response to "35 lewis dot diagram for o2"
Post a Comment