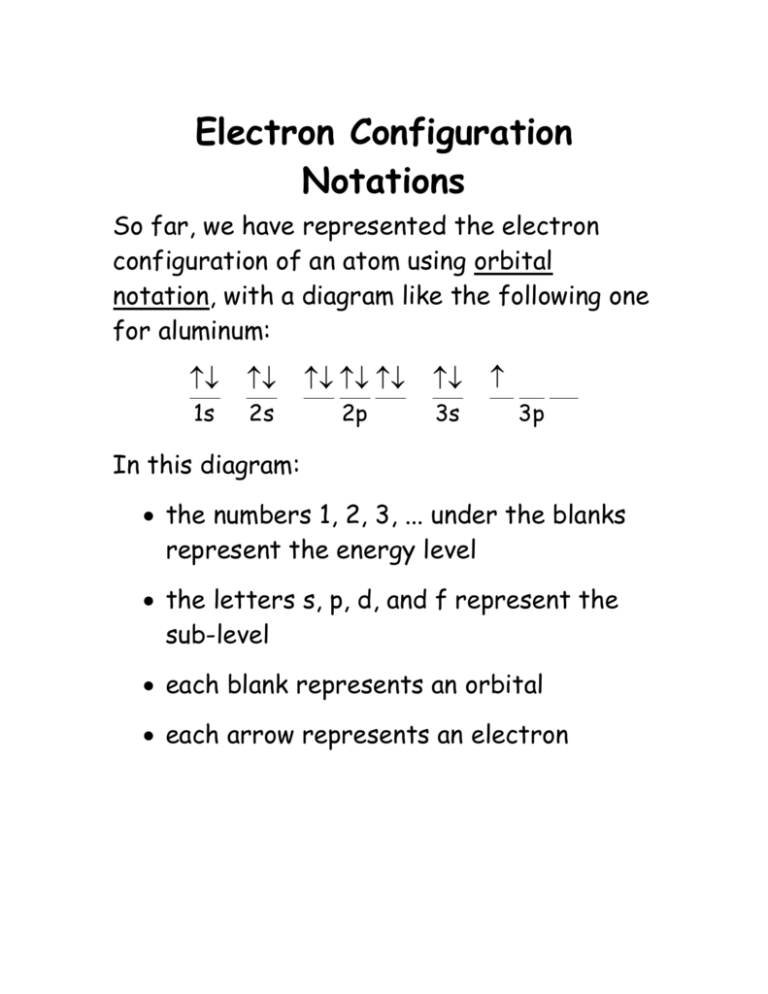
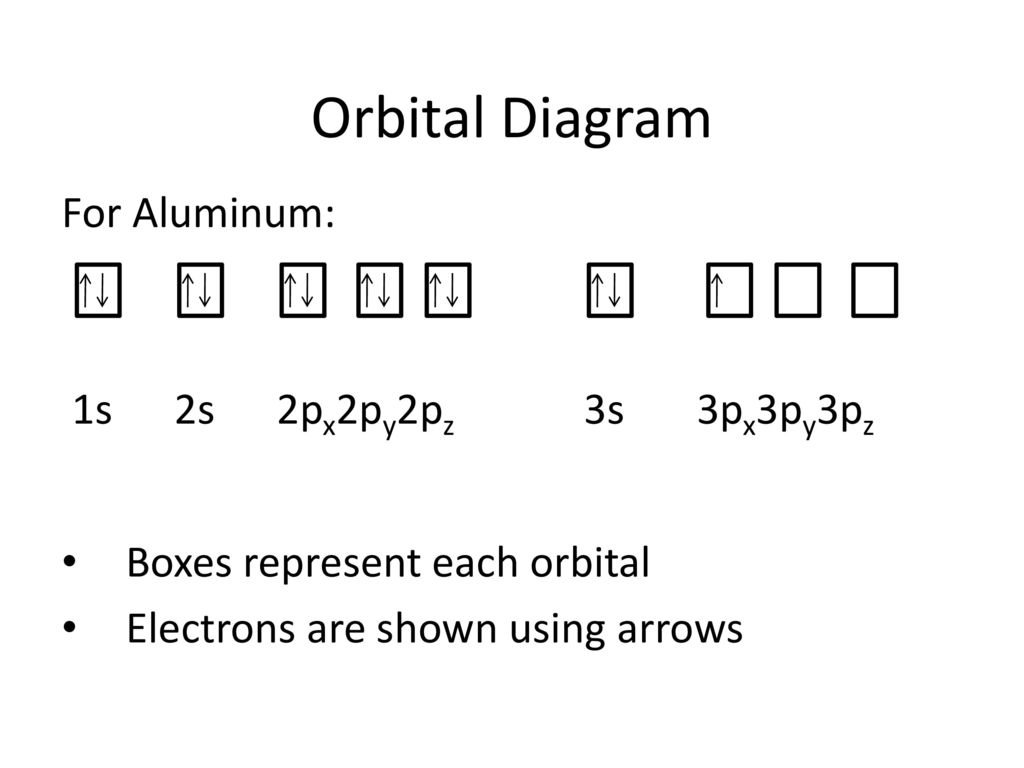
35 orbital diagram for al
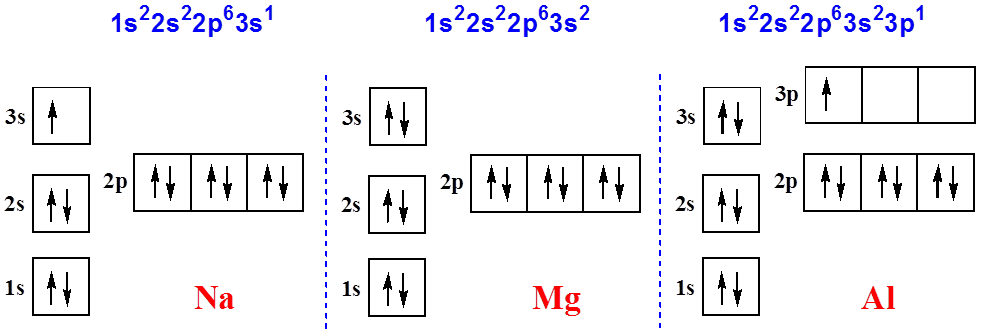
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. Electron Orbital Diagram (Aluminum) Full vs. Condensed Electron Configurations. The full or longhand electron configuration involves going from the 1s orbital and to the last orbital of a specific element. Al (13 Electrons) 1s 2 2s 2 2p 6 3s 2 3p 1. The condensed electron configuration begins at the noble gas just before we reach our specific ...
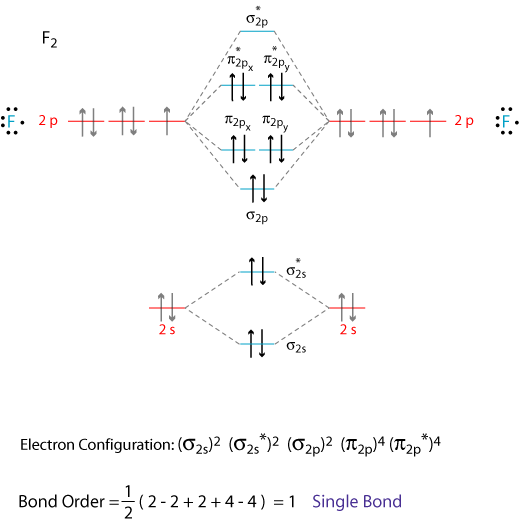
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine

Orbital diagram for al
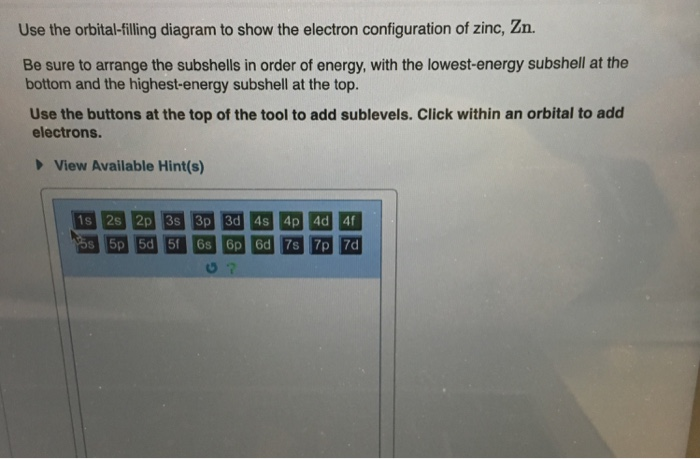
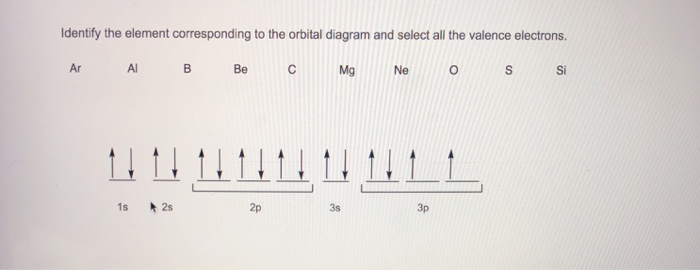
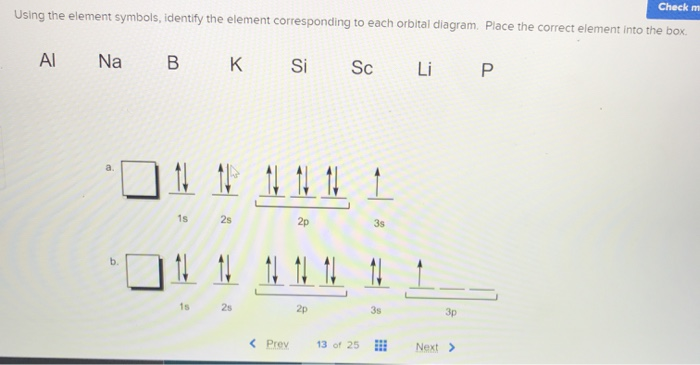
Chemistry questions and answers. Use the electron arrangement interactive to practice building electron arrangements Identify the element that corresponds to the orbital diagram.1s' 252 2p 382 3p O Si O AI O Ne O c 3s廾 2s # is # Create the orbital diagram for sodium. Answer Bank 3s 2p 2s 1s. The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. Choose the orbital diagram that represents the ground state of N. orbital diagram where 1s and 2s orbitals contain 1 pair of electrons each. 2p orbitals are empty. orbital diagram where 1 s and 2 s orbitals contain 1 pair of electrons each. 2 p orbitals contain 3 pairs of electrons.
Orbital diagram for al. Orbital Diagram Configuration Orbital Diagram Configuration . Dr. Mihelcic Honors Chemistry 2013-14 8 Homework Worksheet #7: Electron Configurations ... O Al I F Cf Mg Forms a +3 ion _____ Ends its configuration in the f sublevel _____ Largest atomic radius of all the halogens above_____ Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Which principle or rule is violated by the following orbital diagram of an atom in its ground state? ** the second 2p orbital is empty. ... Al, P, Cl, K. K>Al>P>Cl. Below are data on the first four ionization energies for the fictitious element X First ionization energy= 500kJ/mol Calcium Orbital Filling Diagram. In order to write the Calcium electron configuration we first need to know the we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. The atomic number of calcium is This means that in a neutral calcium atom, there are 20 protons in its nucleus. A neutral calcium atom also.
The following diagram shows the relation between the line of the solstice and the line of apsides of Earth's elliptical orbit. The orbital ellipse goes through each of the six Earth images, which are sequentially the perihelion (periapsis — nearest point to the Sun) on anywhere from January 2 to January 5, the point of March equinox on March 19, 20, or 21, the point of June solstice on June ... Download scientific diagram | Schematic molecular orbital diagrams of Al 2 and AlSi. For simplic- from publication: Electronic structure and photoelectron spectroscopy of AlSi mixed dimer | The ... The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
The molecular orbital diagram representing this order of energy levels is shown in fig. Fig. No. 5 Order of Energy Levels for Boron, Carbon, Nitrogen etc. This kind of energy reversal is due to mixing of 2s and 2p orbitals where the energy difference is very close, that is, for B, C, and N atoms. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... 29-08-2017 · The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
Give the orbital diagram for aluminum. Electron Configuration: To determine the electron configuration and draw an orbital diagram of aluminum, we follow 3 rules:
Orbital diagrams make use of a box, circle, or line for each orbital in the energy level. An arrow is used to represent an electron . and. ... Al has the condensed configuration [Ne]3. s. 2. 3. p. 1 ...
Dr. A. Al-Saadi 21 associated with unpaired electrons. Diamagnetism is associated with paired electrons. Liquid oxygen, O 2(l), is attracted to the poles of a magnet because O2 is paramagnetic. Molecular Orbital Diagram Molecular orbital theory helps Chapter 9 Section 6 Molecular orbital theory helps you predict several important
A condensed (or abbreviated) electron configuration is a way to draw an orbital diagram for a late electron. For this type of diagram the core electrons are replaced with the symbol for the noble ...
In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next ...
01-11-2021 · Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al ...
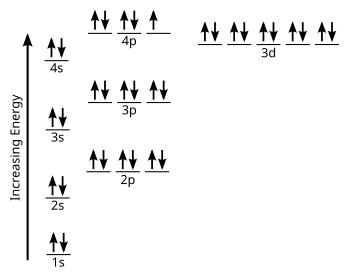
The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital. He (Z = 2): 1s 2. The third electron goes into the next orbital in the energy diagram, the 2s orbital. Li (Z = 3): 1s 2 2s 1
06-11-2021 · Use a qualitative molecular orbital energy-level diagram to predict the valence electron configuration, bond order, and likely existence of the Na 2 − ion. Given: chemical species. Asked for: molecular orbital energy-level diagram, valence electron …
Overview. A molecular orbital (MO) can be used to represent the regions in a molecule where an electron occupying that orbital is likely to be found. Molecular orbitals are approximate solutions to the Schrödinger equation for the electrons in the electric field of the molecule's atomic nuclei.However calculating the orbitals directly from this equation is far too intractable a problem.
"Al"^(3+): 1s^2 2s^2 2p^6 Your starting point here will be the electron configuration of a neutral aluminium atom, "Al". Aluminium is located in period 3, group 13, and has an atomic number equal to 13. This tells you that the electron configuration of a neutral aluminium atom must account for a total of 13 electrons. The electron configuration of the neutral atom looks like this "Al: " 1s^2 ...
For instance, orbital energy diagrams show that the 3d orbital energies are lower than the 4s orbital energies for all known elements with Z > 20. However, most of the elements in the first transition series have electron configurations with one or two electrons in the 4s orbital.
Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
26-09-2021 · This website makes extensive use of JavaScript. The top menus will not function without it and most tools will also not work. If you do not know how to enable JavaScript in your web browser, you should be able find instructions by searching the web for "enable javascript in my browser" (or similar ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
What is the orbital diagram of CO 27? What is the electronic configuration of 27? Which of the following is the electron configuration for the element with the atomic number 27? What is the approximate mass of an atom that contains 27 protons 27 electrons and 32 neutrons? What is the charge of Al 27? How many electrons does al 27 have?
The 2s orbital is shown below, once again represented by a dot density diagram and a boundary surface diagram. Notice how the dot density diagram reveals a feature about the 2 s orbital that boundary surface does not: A node divides the 2 s orbital in two, a portion of the electron cloud is near the center, while another portion lies beyond the ...
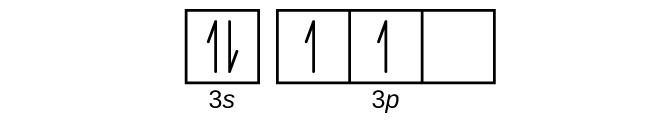
7) The following is a representation of the orbital diagram of Al. What are the four quantum numbers of the electron highlighted in the 3s subshell? 1s 2s 2p 3s 8) Use the periodic table to write the electron configuration for Si. Represent the core electrons with the symbol of the previous noble gas.
The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
Choose the orbital diagram that represents the ground state of N. orbital diagram where 1s and 2s orbitals contain 1 pair of electrons each. 2p orbitals are empty. orbital diagram where 1 s and 2 s orbitals contain 1 pair of electrons each. 2 p orbitals contain 3 pairs of electrons.
The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7.
Chemistry questions and answers. Use the electron arrangement interactive to practice building electron arrangements Identify the element that corresponds to the orbital diagram.1s' 252 2p 382 3p O Si O AI O Ne O c 3s廾 2s # is # Create the orbital diagram for sodium. Answer Bank 3s 2p 2s 1s.





















0 Response to "35 orbital diagram for al"
Post a Comment