35 square planar splitting diagram
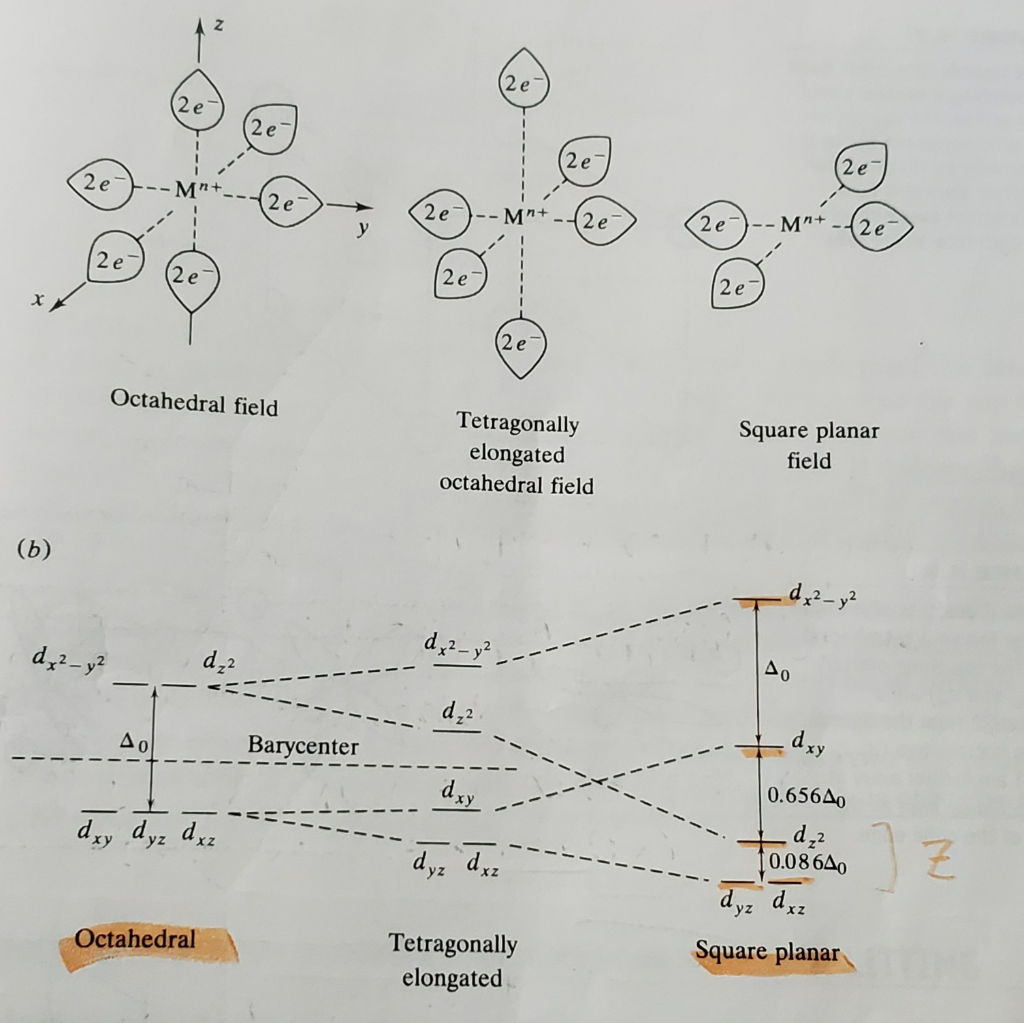
Square planar coordination can be imagined to result when two ligands on the z-axis of an octahedron are removed from the complex, leaving only the ligands in the x-y plane. As the z-ligands move away, the ligands in the square plane move a little closer to the metal. The orbital splitting diagram for square planar coordination can thus be ... e.g. for the square planar structure here discussed, of D 4h symmetry, the rotational operations groupedbyclassare 2C 4 (C 4 andC 4 3),C 2 (collinearwith C 4) ’ d ’’ The C 2 'and C 2 " axes of a planar MX 4 molecule. 2C 2,and 2.
Therefore, the crystal field splitting diagram for square planar geometry can be derived from the octahedral diagram. The removal of the two ligands stabilizes ...
Square planar splitting diagram
by J Börgel · 2016 · Cited by 21 — The splitting of the d-orbital based MOs for square planar complexes with π-acceptor ligands such as cyanide is qualitatively the same as for ... 7 May 2021 — We find that the square planar complexes have the greatest crystal field splitting energy compared to all the other complexes. This means that ...Electrons in Orbitals · Description of d-Orbitals · Square Planar Complexes Octahedral CFT splitting. Electron diagram for octahedral d shell splitting. Crystal field stabilization is applicable to the transition-metal complexes of all geometries. The reason that many d 8 complexes are square-planar is the very large amount of crystal field stabilization that this geometry produces with this number of electrons.
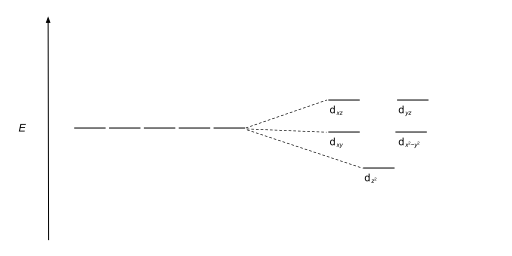
Square planar splitting diagram. D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ... The crystal field splitting diagram for a square pyramidal crystal field is given below. Through considerations similar to those employed in class for octahedral and square planar geometries, assign each energy level to an appropriate d orbital and explain which d orbital is the most destabilized in the square pyramidal crystal field. The most basic crystal field argument includes point-symmetric charges approaching the central metal in a way as the ligands would. Then, any orbitals that are symmetry-equivalent will end up at the same energy, and depending on how much these point towards the point-symmetric approaching charges they will be raised or lowered. Square Planar Complexes Consider a CFT diagram of a tetragonal elongation taken to its extreme: tetragonal elongation removal of z ligands eg t2g b2g dxydxzdyz eg dz2 dx2-y2 dxzdyz dxy dz2 dx2-y2 a1g b1g b2g eg dxzdyz dxy dz2 dx2-y2 a1g b1g ∆1,sp Octahedral Square Planar Δ> Π
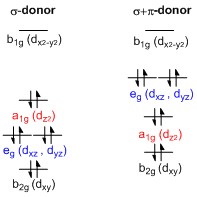
A general d-orbital splitting diagram for square planar (D 4h) transition metal complexes can be derived from the general octahedral (O h) splitting diagram, in which the d z 2 and the d x 2 −y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy, d xz and d yz orbitals. Consequently, the d x2-y 2 remains unoccupied ... Square planar complexes have a four tiered diagram (i.e. four different sets of orbitals with different energies). If it has a two tiered crystal field splitting diagram then it is tetrahedral. But this assumes you have the crystal field splitting diagram of the complex. The octahedral ion [Fe(NO2)6]3−, which has 5 d-electrons, would have the octahedral splitting diagram shown at right with all five electrons in the t2g level. Square Planar D Orbital Splitting Diagram. Crystal Field Theory (CFT) is a model that describes the breaking of degeneracies of electron In a tetrahedral crystal field splitting, the d-orbitals again split into two groups, with an energy difference of Δtet. The lower energy Square planar and other complex geometries can also be described by CFT.
Figure 2. Splitting of the t2g set and the eg set of orbitals in a square planar crystal field. The crystal field ... Octahedral CFT splitting. Electron diagram for octahedral d shell splitting. Crystal field stabilization is applicable to the transition-metal complexes of all geometries. The reason that many d 8 complexes are square-planar is the very large amount of crystal field stabilization that this geometry produces with this number of electrons. 7 May 2021 — We find that the square planar complexes have the greatest crystal field splitting energy compared to all the other complexes. This means that ...Electrons in Orbitals · Description of d-Orbitals · Square Planar Complexes by J Börgel · 2016 · Cited by 21 — The splitting of the d-orbital based MOs for square planar complexes with π-acceptor ligands such as cyanide is qualitatively the same as for ...
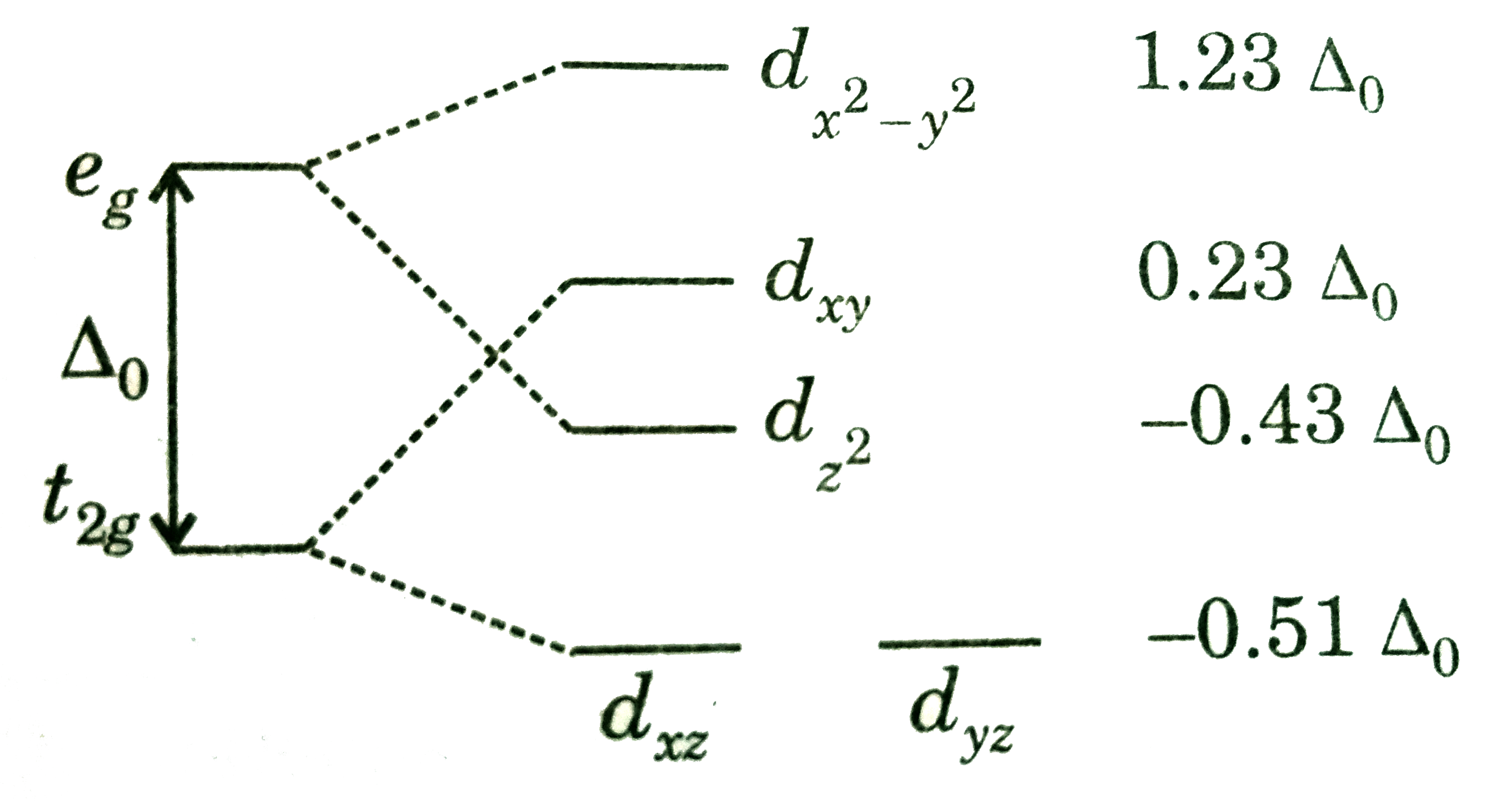
Related Videos Of The Splitting Diagram For Square Planar Complexes Is More Complex Than For Octahedral And Tetrahedral Complexes And Is Shown Below With The Relative Energies Of Each Orbital Br Img

Show By Means Of A Diagram How The Pattern Of D Orbital Splitting Changes As An Octahedral Complex Undergoes Tetragonal Distortion And Eventually Becomes A Square Planar Complex

What Does The Crystal Field Splitting Diagram For Trigonal Planar Complexes Look Like Chemistry Stack Exchange

The Splitting Diagram For Square Planar Complexes Is More Complex Than For Octahedral And Tetrahedral Complexes And Is Shown Below With The Relative Energies Of Each Orbital Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images














0 Response to "35 square planar splitting diagram"
Post a Comment