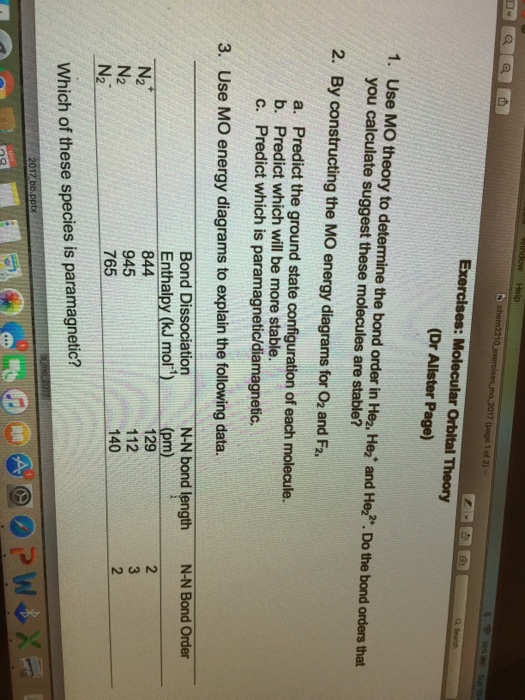
36 calculate bond order from mo diagram
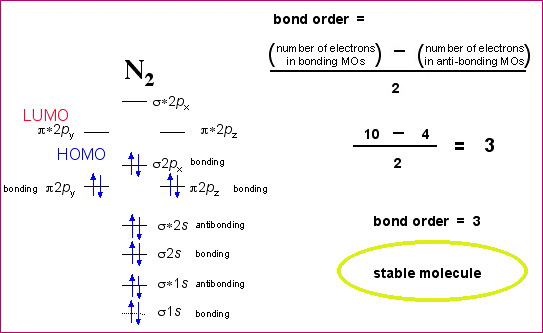
Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons. Click here 👆 to get an answer to your question ️ How to calculate bond order from molecular orbital diagram? halexisjennah halexisjennah 02/13/2017 Chemistry High School answered How to calculate bond order from molecular orbital diagram? 1 See answer Advertisement
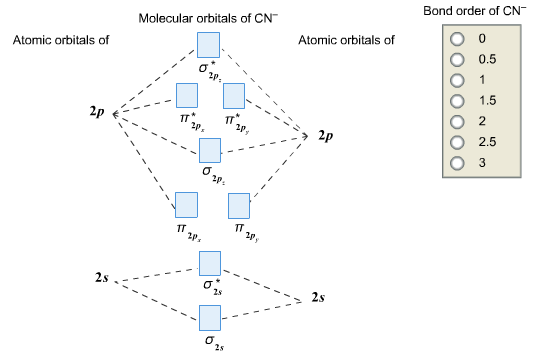
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
Calculate bond order from mo diagram
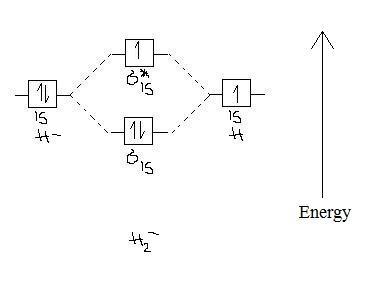
Bond order is also an index of bond strength, and it is used extensively in valence bond theory. Dihydrogen (H 2) This MO diagram depicts the molecule H 2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely occupied. A bond order of one is obtained by employing the formula above ... 📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at http://conquerchemistry.com/chem-secrets/💯 If you like my teaching style and are inte... Bond Order in Molecular Orbital Theory — The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (1-0)/2 ...
Calculate bond order from mo diagram. Click here to get an answer to your question ✍️ Draw the molecular orbital diagram of dioxygen and calculate bond order.1 answer · Top answer: Bond order = [ No. of electrons in bonding orbitals ] - [ No. of elevtrons in antibonding orbitals ]2 = 10 - 62 = 42 = 2 Bond order of Oxygen (O) is 2 . O = O A bond order of one is obtained by employing the formula above indicating a stable bond. Bond orders can be calculated from lewis structures which are the heart of the valence bond model. Lecture 10 Part B Mo Diagram Of Nh3 Youtube Bond order no. How to calculate bond order from mo diagram. Molecular orbital mo theory and the bond order. Bond order formula - molecular orbital theory — The molecular orbital theory has never been so clear as with our bond order calculator. In the ... Use the MO diagram (below) to calculate the bond order for BN. ор 0 ; Question: Use the MO diagram (below) to calculate the bond order for BN. ор 0 . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer.
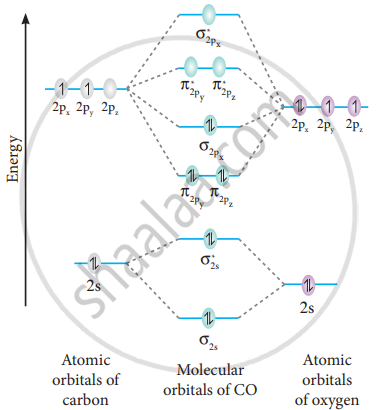
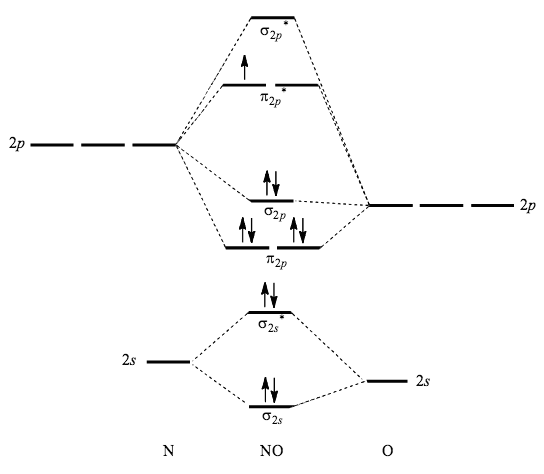
In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; ... Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ... 4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3. We'll need details about the molecule's molecular orbital diagram to figure out the bond sequence. The electrons are present in bonding orbitals and which are ...1 answer · Top answer: Hint: A molecular orbital is a mathematical feature in chemistry that describes the position and wavelike activity of an electron in a molecule. This ...
The bond order of the formed bond can be calculated from the MO diagram as follows: bond order = (number of bonding electrons)- (number of anti-bonding electrons) 2 bond order = (number of bonding ... Bond Order in Molecular Orbital Theory — The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (1-0)/2 ... 📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at http://conquerchemistry.com/chem-secrets/💯 If you like my teaching style and are inte... Bond order is also an index of bond strength, and it is used extensively in valence bond theory. Dihydrogen (H 2) This MO diagram depicts the molecule H 2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely occupied. A bond order of one is obtained by employing the formula above ...

Explain The Calculation Of Bond Order In Be Molecule On The Basis Of Molecular Orbital Theory Brainly In

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com





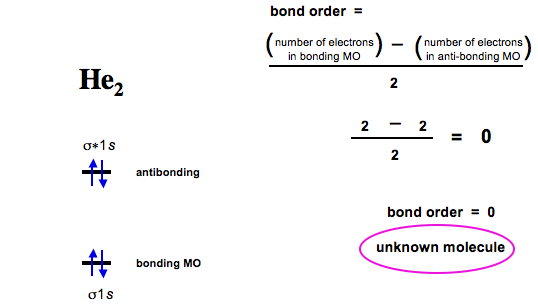

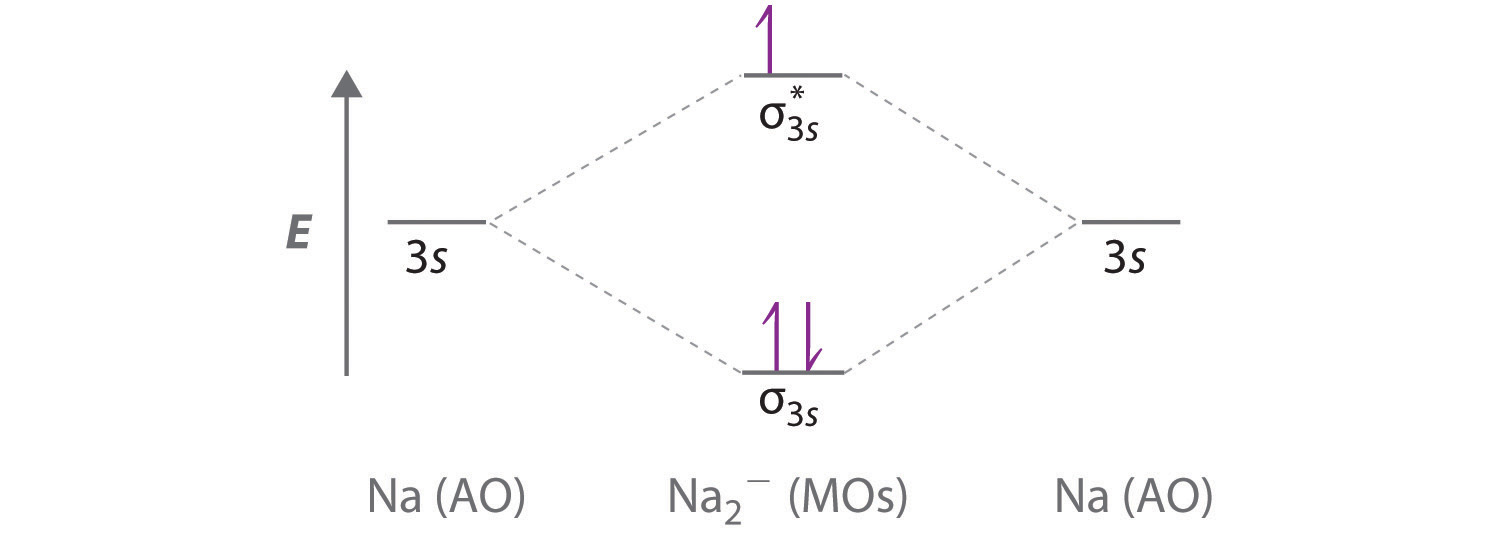
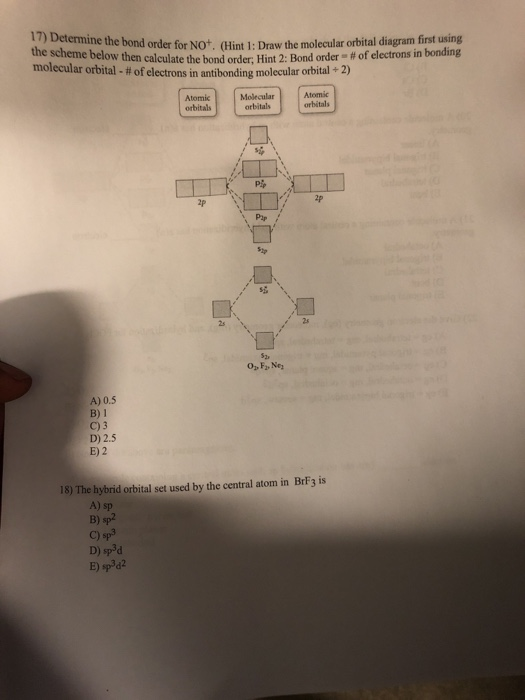

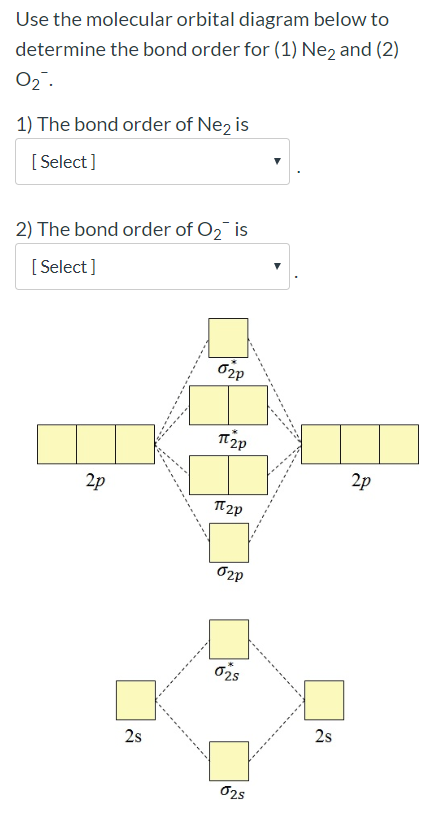











0 Response to "36 calculate bond order from mo diagram"
Post a Comment