36 ch4 electron dot diagram
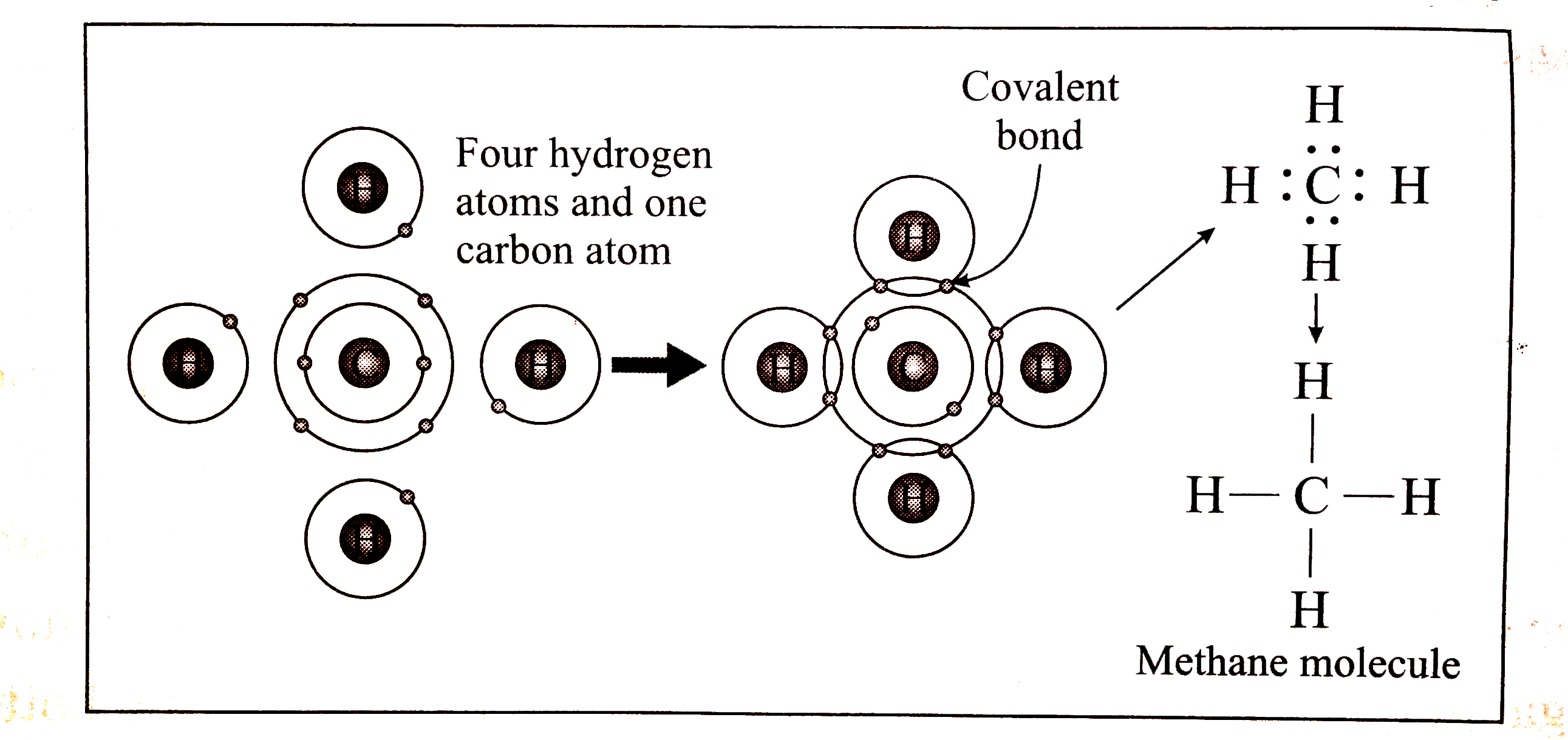
CH4 Lewis Structure Lewis Structure for CH4 (Methane). Within the CH₄ (methane) Lewis Dot structure, we must first find the valence electrons of carbon and hydrogen. We express the valence electrons as points within the Lewis point structure. To induce carbon's valence electrons, we must appear within the electronic configuration of carbon. ... November 28, 2017 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
Transcript: For the CH4O Lewis structure, we have 14 valence electrons. So there are a number of ways to draw the Lewis structure for CH4O in which each of the atoms has a full outer shell and we only use 14 valence electrons. So I've drawn three here. When I look at these structures, my experience ...

Ch4 electron dot diagram
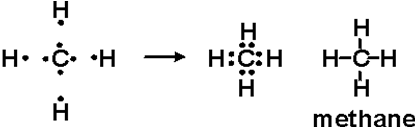
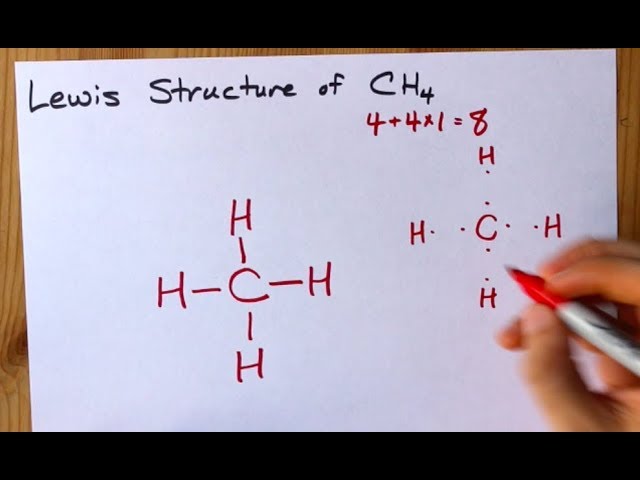
December 26, 2019 - Click here👆to get an answer to your question ✍️ Draw the electron dot diagram of CH4 and justify your answer. March 9, 2017 - Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the... A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...
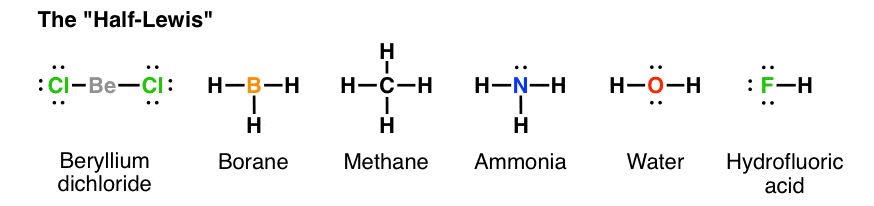
Ch4 electron dot diagram. So, we can form the electron dot diagram for $C{H_4}$ as follows: Here, the valence electrons of the carbon atom are represented by dot and valence electron of H-atom is represented by *. So, we can clearly see that one electron of carbon and one electron of hydrogen atom combine to form a ... CH4 Lewis Structure. Lewis framework is the pictorial representation that the plan of valence shell electrons in the molecule, which help us recognize the atoms' link formations. The electron that participate in bond formation are dubbed the bonding pair the electrons, if those that don't are known as nonbonding bag of electrons. Woodbank Communications Ltd Woodbank, South Crescent Road , Queens Park Chester, CH4 7AU United Kingdom. ... Components inside the dotted rectangle ... Draw the electron dot diagram of chemical bonds in methane `(CH_4)` and ethane`(C_2H_6)`. Draw the electron dot diagram of chemical bonds in methane `(CH_4)` and ethane`(C_2H_6)`. Books. Physics. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Chemistry. NCERT P Bahadur IIT-JEE ...
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. ... to denote “ electron-poor ” and “ electron-rich ... Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots 1 Answer Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in BF3. Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule. Use the VSEPR shape to determine the angles between the electron domains. Do you need help with your homework? On Toppr Answr you can scan any question, and get its answer instantly
Which of the following is/are not the condition(s) for Lewis dot structure? (i) Each bond is formed as a result of sharing of an electron pair between the atoms. (ii) From the two combining atoms only one atom contribute electron(s) to the shared pair. ... CH 4 (c) CO 2 (d) NO. Answer. D. To draw the lewis Dot structure of CH₄(methane), we have to find out the valence electrons of carbon and hydrogen first.We express valence electrons as dots in lewis dot structure. How … In fact the molar mass of methane is so minuscule that it is sometimes mentioned as a possible lifting gas because its density is less than that. is methane ionic or covalent, _____4. 15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2… CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!

Electrons Ck Foundation The Lewis Dot Formula Electron Dot Structure Of Oxygen Clipart 565920 Pinclipart
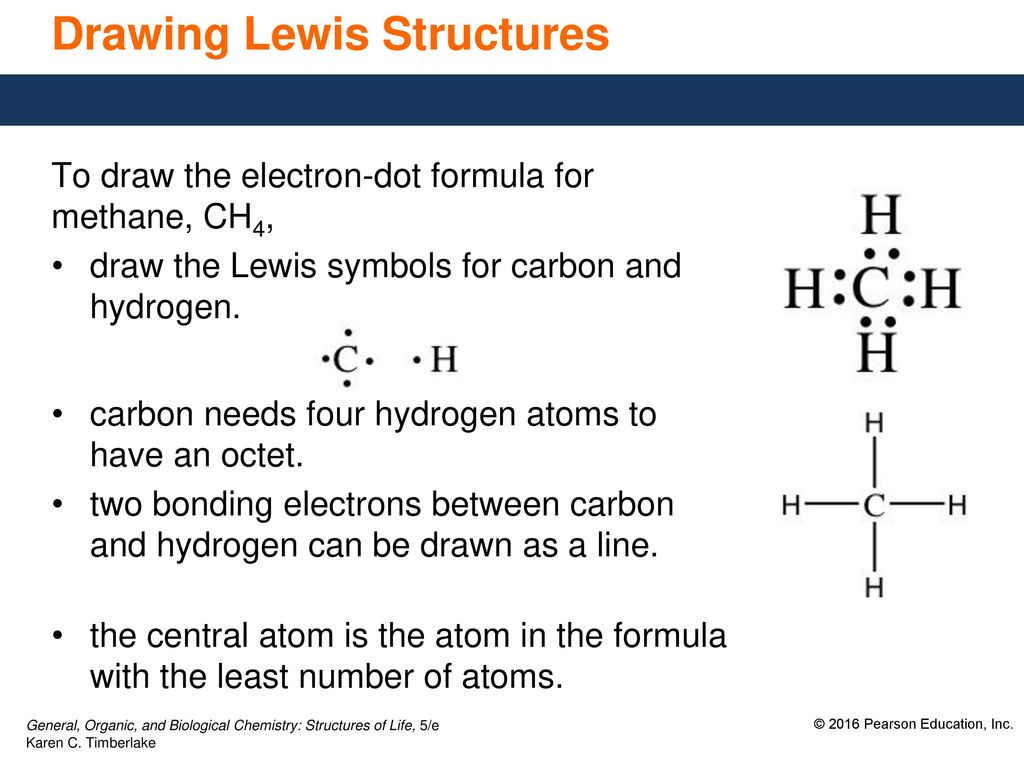
Explains how to draw the lewis dot structure for ch 4 methane. Drawing the Lewis structure for CH4(named methane) requires only single bonds. Lewis dot symbols and lewis structures. Lewis Electron Dot Structures. In the case of n-butane, all carbon atoms are in straight-chain whereas, in the case of isobutane, there is a side chain in the molecule.
Mar 25, 2021 · to know about the polarity of the ch4 molecule, check out our detailed blog post on ch 4 polarity to find out if the molecule is polar or nonpolar. In this article, we will discuss phosphorous trifluoride (pf3) lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, etc.
January 10, 2019 - Step 5: The rest are nonbonding pairs. Subtract bonding electrons (step 3) from valence electrons (step 1). ... Use information from step 4 and 5 to draw the CH4 lewis structure. ... Alternatively a dot method can be used to draw the CH4 Lewis structure.
Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule which helps us understand the atoms bond formations. Lewis dot structure of CH 4. CH4 Lewis Structure. Knowing the arrangement of atoms distribution of electrons and the shape of the molecule is vastly important in chemistry.
Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4.
Ch4 Polar Or Nonpolar : Chemical bonding 1. In this article, we will discuss methane (ch4) lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, . Molecules which have polar bonds that cancel each other out because the molecule is symmetrical around a central atom.
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. Read full answer.
© 2016 Houston Community College. All rights reserved · About | Contact | Support
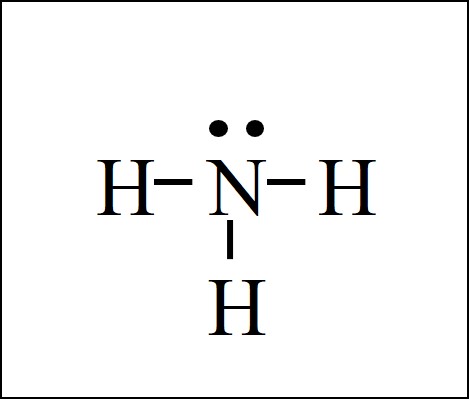
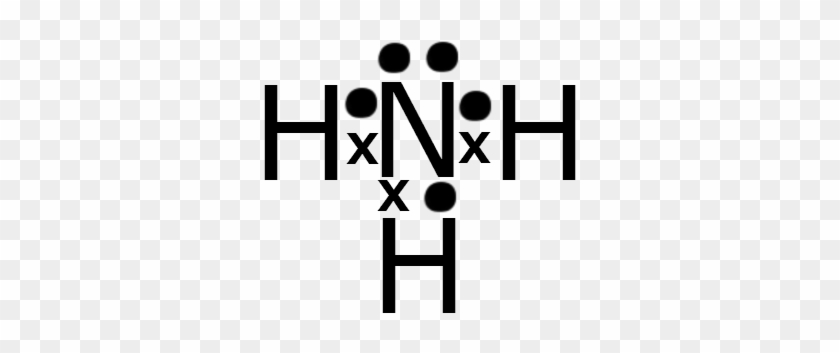
The Repulsive Effect of the Lone Pair Electrons. The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O.
After discussing Lewis-dot notation and formal charge, Professor McBride shows that in some " single- minimum " cases the Lewis formalism ...
Explain The Bonding In Methane Molecule Using Electron Dot Structure Sarthaks Econnect Largest Online Education Community
molecular dot diagrams and determine shape,bond angle and molecule polarity ... What are the steps/rules to drawing molecular dot diagrams? How do ...

In The Space Provided Below Draw Electron Dot Diagrams For The Following Molecules Hydrogen H2 Brainly Com
Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
CH4 Lewis structure Methane electron dot structure is that type of diagram where we show the total 8 valence electrons of CH4 as dots or dots and dashes -In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots. A Estructura electrónica de Lewis.
Lewis structure helps with understanding the placement of atoms in. Lewis dot structure of C 2 H 6. Three are two ways to draw the lewis structure for c2h6o. What is the Lewis structure for C2H6. This means that the Lewis dot structure for C2H 6 must account for 14 valence electrons either through bonding between atoms or through lone pairs.
US5394292A - Electronic car bumper - Google Patents ... 5 is a circuit diagram showing an example of the present invention;

Methane Molecule Ch4 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes
Answer: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Methane’s (When it comes to hydrocarbons, “meth” stipulates one carbon, “ane” stipulates a single bond shared with hydrogens) molecular ...
Answer The Following Question Explain The Bonding In Methane Molecule Using Electron Dot Structure From Chemistry Chemical Bonding Class 10 Icse
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds I Methane Sarthaks Econnect Largest Online Education Community
Interfacing MQ2 to Arduino- Gas Sensor for Smoke-Butane-CH4 and LPG jojo February 23, 2016 11 Comments In this article, we are going to learn how to ...
... Zener diode was added to , : No TVS Zener Diode , 24-V Soft Start-up Test One: No TVS Zener Diode , 24-V Soft Start-up Figure 2 , , CH3 10 V/div, CH4 ...
Draw and interpret simple electron dot-and-cross diagrams to show how atoms bond through ionic, covalent and dative covalent bonds and be able to ...
The AsF3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the AsF3 molecule. The geometry of the AsF3 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the AsF3 geometrical shape in which the ...
From the above reaction, in the dot structure of methyl chloride (CH3Cl) there are four pairs of shared electrons between carbon and other atoms. Each pair of shared electrons constitutes one single covalent bond. So, methyl chloride has four single covalent bonds. Question 12 Draw the electron dot structures for-Solution:

Im Dunkeln Leuchten Lewis Dot Diagramme Senior Chemie Chemie Chemie D Chemie Dot Organic Chemistry Study Teaching Chemistry Chemistry Lessons
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
10+ Ocl2 Lewis Structure. So we've used all 20 valence electrons for the ocl2 lewis structure. 8.34) use lewis symbols and lewis structures, diagram formation of pf3. Model Kit CH4 - YouTube from i.ytimg.com For the socl2 lewis structure we first count the valence electrons for the socl2. Experiment 9…
I quickly take you through how to draw the Lewis Structure of methane, CH4. I also go over hybridization, shape and bond angle.
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Give The Electron Dot Structures Of I Nacl Ii Mgcl2 Iii Cao Sarthaks Econnect Largest Online Education Community
If you change the 2 in front of 2O, to a 3, the result of the right side of the equation is . Given the chemical equation expressed as:. From the equation, we can see that 1 mole of methane reacts with 2 moles of oxygen to form 1 mole of carbon dioxide and 2 moles of water.. If the number of moles of oxygen is replaced with 3 moles, the correct equation will be expressed as:
A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like.
Check me out: http://www.chemistnate.com
Step by step explanation showing how to draw the Lewis structure of Methane (CH4).
In the BrO3- Lewis structure Bromine (Br) is the least electronegative and goes in the center of the dot structure.
What is the Lewis dot structure for CCl4? Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetrical geometry, CCl 4 is non-polar.
The calculation for methane shows that the carbon atom is associated with 8 electrons in the σ framework. This corresponds to four shape-determining electron pairs. The coordination geometry of carbon is consequently tetrahedral. There are four bonded groups, therefore there are no lone pairs ...
May 18, 2020 - The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only ...


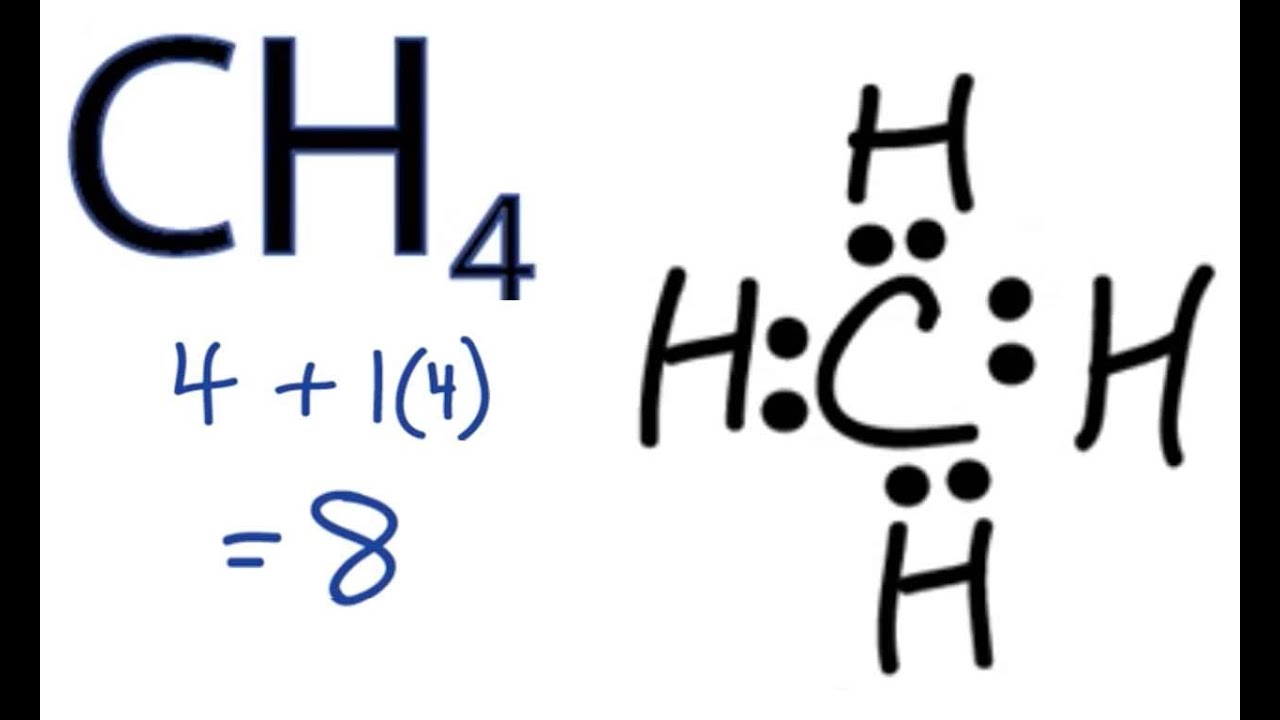











0 Response to "36 ch4 electron dot diagram"
Post a Comment