36 newman projection energy diagram
Newman projection of butane energy diagram Organic Chemistry Reactions, Chemistry Classroom, Chemistry Lessons,. chemistrysteps. Chemistry Steps. Transcribed image text: Using Newman projections and an energy diagram, draw all of the conformers of 1,1,2- tribromoethane and indicate the energy changes that arise from rotation about the C-C bond. You do not need the actual energy values, but you should label relative energies (energy minima and maxima).
in the video on sp3 hybridised orbitals we went in pretty good detail about how a methane molecule looks but just as a bit of review it's a tetrahedral shape you have a carbon in the middle and then you would have a hydrogen you could imagine I'm drawing it like this because this hydrogen is poking out of the page then maybe you have another hydrogen that's in the page you have one above the ...

Newman projection energy diagram
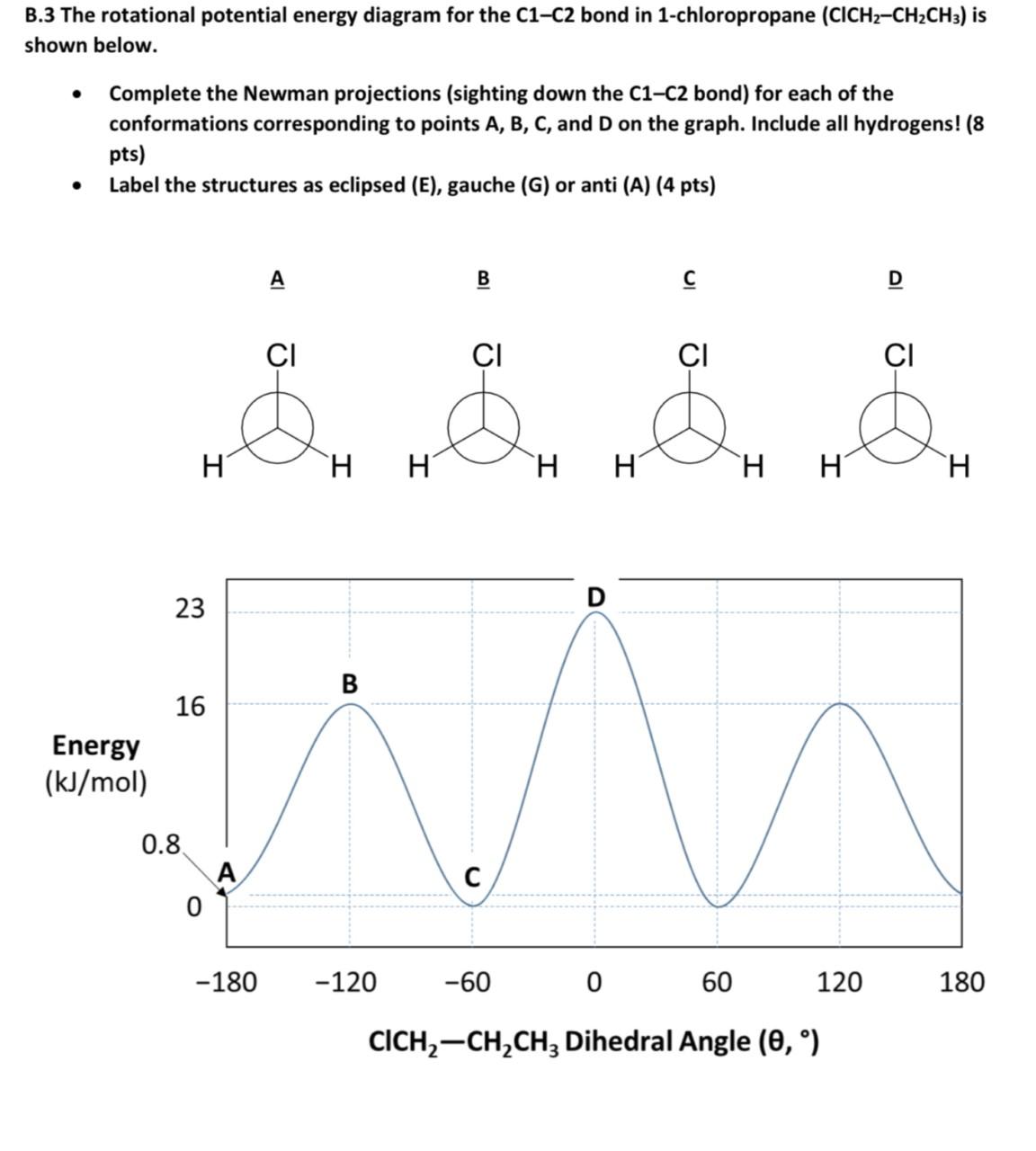
Newman’Projection’Practice’ 3’ D. Newman Projection Energy Diagrams. 14. Draw a qualitative energy diagram for CH 3CH 2CH 2CH(CH 3) 2, relative to the bond between the two CH2 carbons. The Newman projections are drawn below, using “iPr” as an abbreviation for the isopropyl CH(CH 3) 2 group. Put “S” (for staggered) by any ... Newman projection, relative to the bond indicated in each question. The most stable conformations will be staggered conformations with the largest groups ANTI to each other. The least stable, highest energy conformation will have an eclipsed conformation with the largest groups sterically overlapped on top of each other. a. butane, C2-C3 bond Cyclohexane Newman Projections. Cyclohexane chair conformations can also be portrayed through a Newman projection, but it’s a little bit different. We actually use what amounts to two Newman projections stuck together, and we call it a double Newman. Let’s draw one for (1R,2R,4S)-4-chloro-2-iodo-1-methylcyclohexane. Cyclohexane Newman
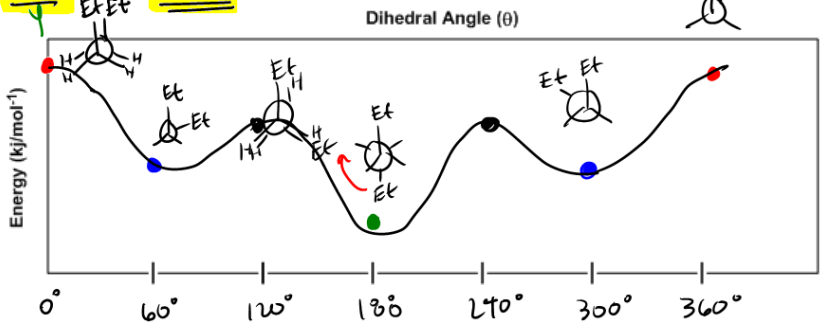
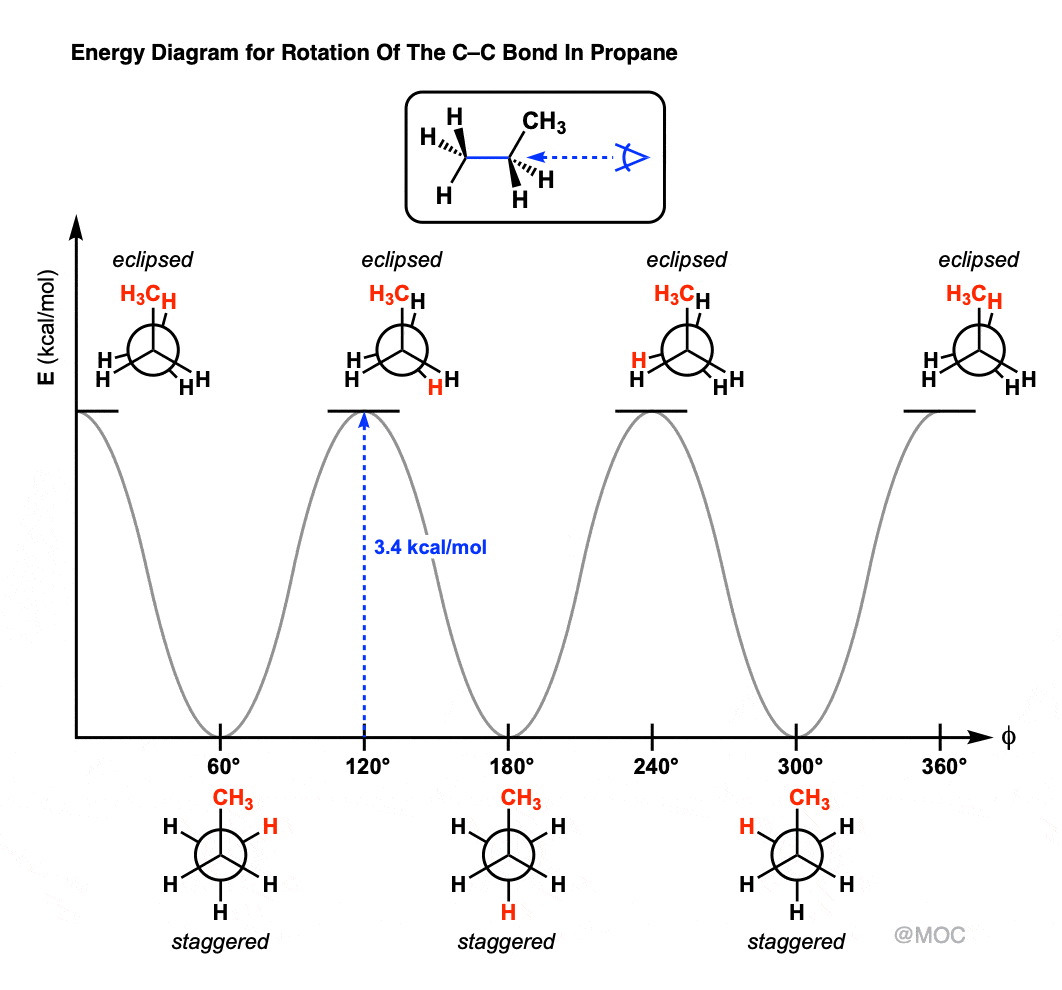
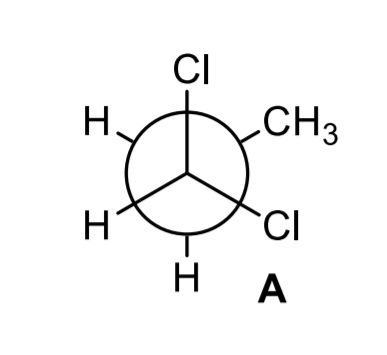
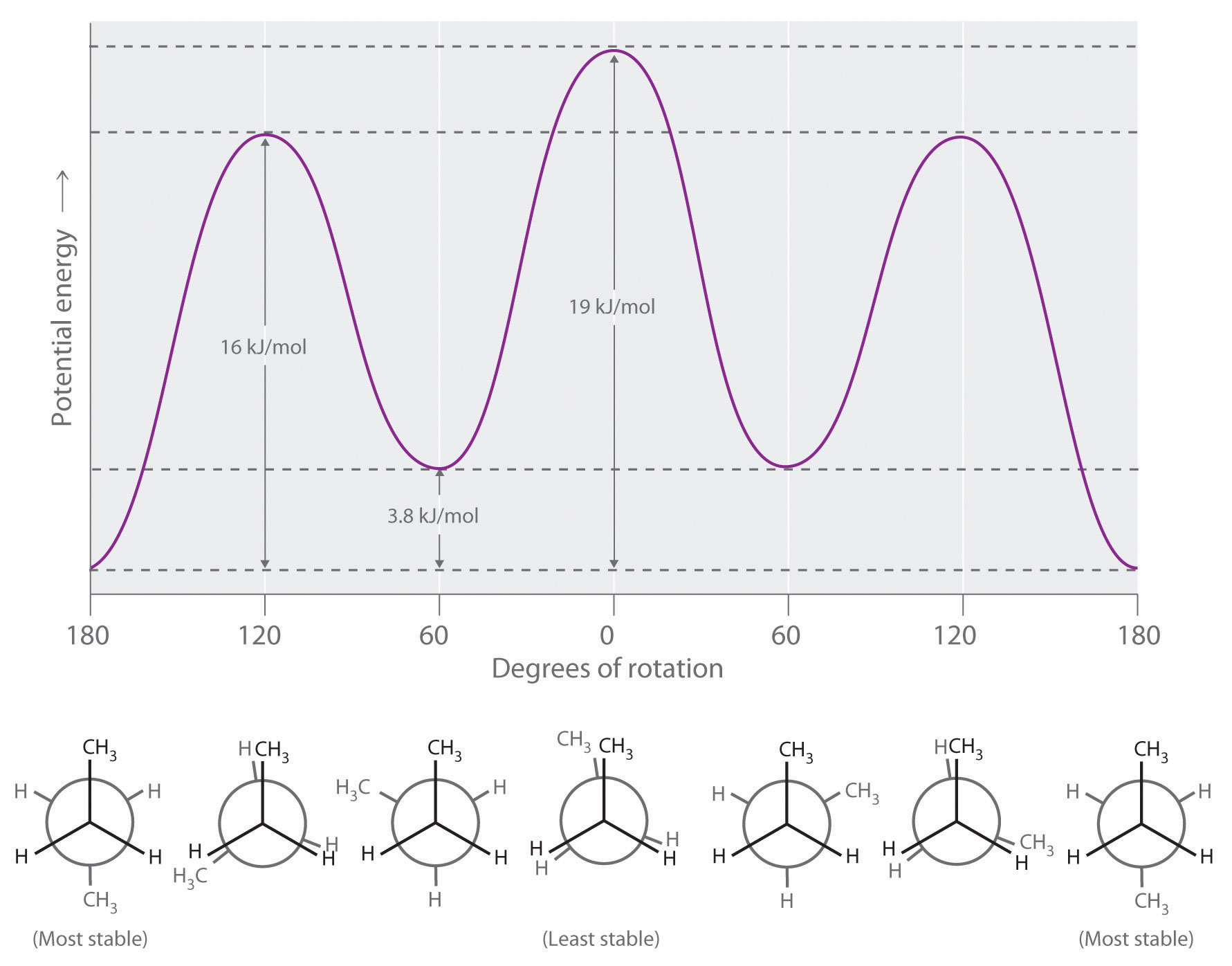
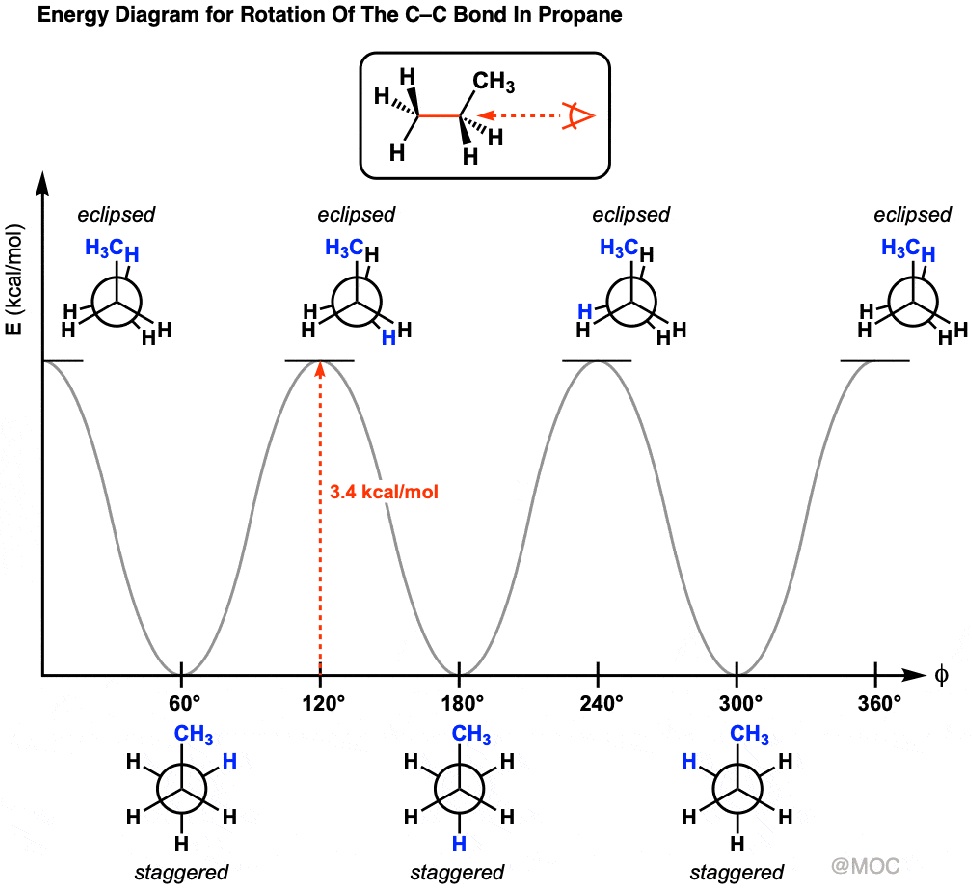
Newman projection energy diagram. 5 Jun 2019 — In order to better visualize different conformations of a molecule, it is convenient to use a drawing convention called the Newman projection. A Newman projection is a representation of the molecule looking through a C-C single bond. For every Newman projection, you need to specify the bond and the direction you are looking at. For example, for our molecule, we can look through the C1-C2 bond (even though it can be through any bond). The direction is usually shown with an eye symbol: Draw the Newman projections for pentane looking down the C2-C3 bond through a full 360 degree rotation. Start with the anti-staggered Newman projection then draw and label each Newman projection (staggered, eclipsed, gauche, anti-staggered, fully-eclipsed) for every 60 ... explain why there is a large energy difference between the two. Essentially, large groups want to be as far apart as possible, but in a Newman projection, the atoms are forced to be fairly close to one another, so there is a repulsive force present. The overlap, and the energy difference associated with this overlap, leads to two energetic subgroups of Newman projections: eclipsed and staggered.
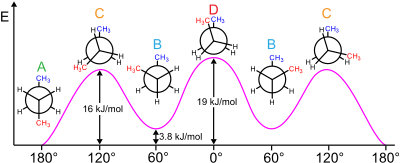
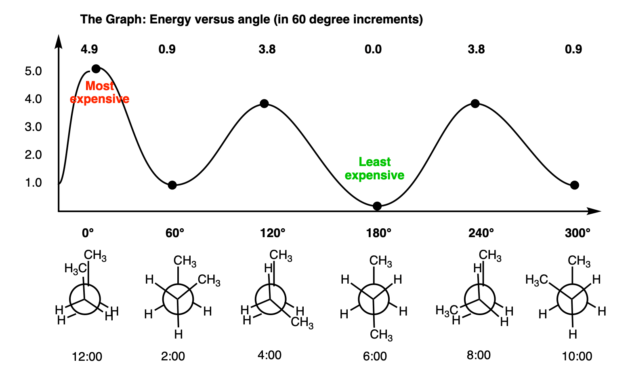
29 May 2020 — The Conformational Isomers (and Newman Projections) of Butane ... We can even graph the energy of the different conformations in 60 degree ... The Newman Projection is an important skill for every organic chemistry student. Not only does it provide you with another perspective of organic compounds, but it also gives you a means to analyze the conformational energy of a molecule. In this article I will give you a quick introduction to the energy diagram of a Newman Projection. Minimum Energy Conformations of 1,2-Dibromoethane For 1,2-dibromoethane, MarvinSketch finds three staggered conformations, as shown. Each structure has been oriented in order to present a view comparable to a Newman projection. The energy is listed above each conformation. The lowest energy conformation is Cyclohexane Newman Projections. Cyclohexane chair conformations can also be portrayed through a Newman projection, but it’s a little bit different. We actually use what amounts to two Newman projections stuck together, and we call it a double Newman. Let’s draw one for (1R,2R,4S)-4-chloro-2-iodo-1-methylcyclohexane. Cyclohexane Newman
Newman projection, relative to the bond indicated in each question. The most stable conformations will be staggered conformations with the largest groups ANTI to each other. The least stable, highest energy conformation will have an eclipsed conformation with the largest groups sterically overlapped on top of each other. a. butane, C2-C3 bond Newman’Projection’Practice’ 3’ D. Newman Projection Energy Diagrams. 14. Draw a qualitative energy diagram for CH 3CH 2CH 2CH(CH 3) 2, relative to the bond between the two CH2 carbons. The Newman projections are drawn below, using “iPr” as an abbreviation for the isopropyl CH(CH 3) 2 group. Put “S” (for staggered) by any ...

Organic Chemistry Lesson Of The Day The 4 Conformational Isomers Of Butane The Chemical Statistician
Solved 25 Which Newman Projection Of Butane Corresponds To The Indicated Position On The Energy Diagram Below Potential Energy Cumstances 60 Course Hero

Importance Of Thorough Conformational Analysis In Modelling Transition Metal Mediated Reactions Case Studies On Pincer Complexes Containing Phosphine Groups Sciencedirect

Newman Projection Of Butane Energy Diagram Organic Chemistry Organic Chemistry Study Chemistry Lessons
Solved Step 2 Identify The Newman Projections For Each Rotation The Potential Energy Diagram Will Look At The Energy Levels As The Carboncarbon B Course Hero

Consider 1 Bromopropane Ch3ch2ch2br A Draw A Newman Projection For The Conformation In Which Ch3 Brainly Com













0 Response to "36 newman projection energy diagram"
Post a Comment