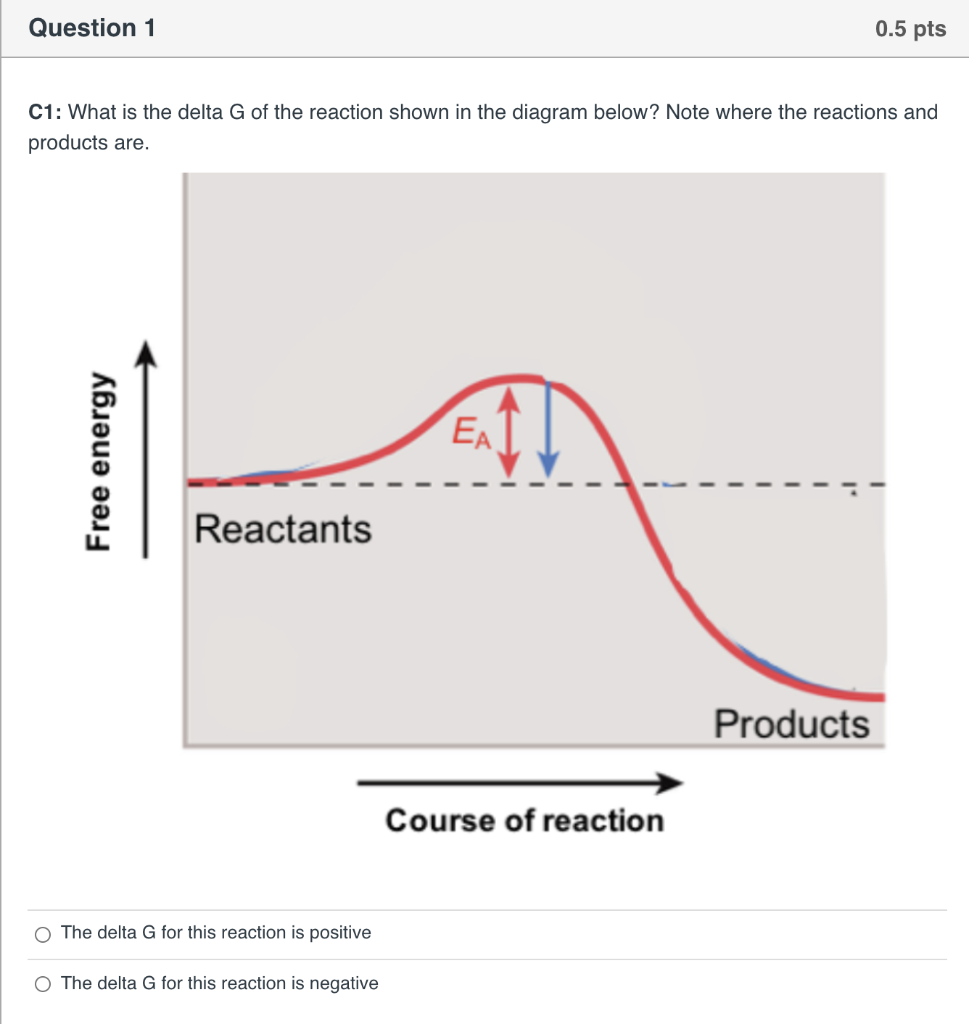
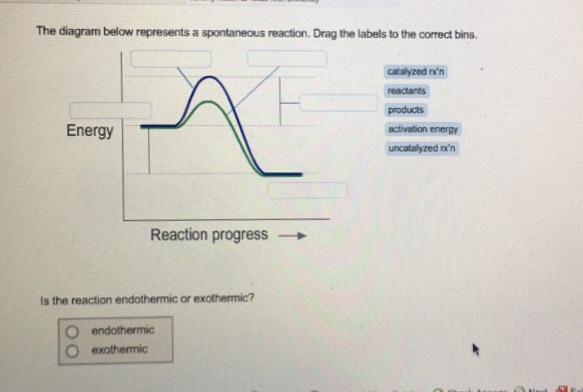
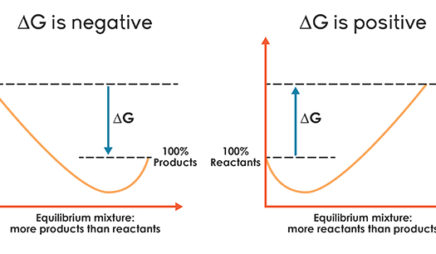
37 the diagram below represents a spontaneous reaction (δg
C 2 H 6 ( g) H 2 ( g) + C 2 H 4 ( g) Answer: Δ G ° = 102.0 kJ/mol; Δ G ° = 102.0 kJ/mol; the reaction is nonspontaneous ( not spontaneous) at 25 °C. The standard free energy change for a reaction may also be calculated from standard free energy of formation ΔGf° values of the reactants and products involved in the reaction. Diagram 2, because it represents a reaction with a high activation energy barrier for molecules to overcome and a very slow reaction rate, even if it is thermodynamically favorable with ΔG < 0 Diagram 2, because it represents a reaction that is thermodynamically favorable with ΔH < 0 the products formed are unstable and quickly revert to form ...
The reaction is associated with a decrease in the number of particles in the same phase (3 mol→2 mol), so overall, a decrease in entropy: ΔS ⦵ for the reaction is negative. Hence, applying ΔG ⦵ = ΔH ⦵ ⦵, ΔG ⦵ will be negative (ΔG ⦵ <0), and the reaction spontaneous, if ΔH ⦵ >TΔS ⦵.

The diagram below represents a spontaneous reaction (δg
chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so Δs will be positive as Δh = Δg tΔs this means that Δh Δg for the other reactions there is a decrease in the number of moles of has so Δs is negative Δh = Δg tΔs this means that Δh Δg These are included in an equation to tell you whether a reaction happens: ΔG = ΔH - TΔS Depending on the combination of signs you have for these two state functions, you'll be able to determine whether a reaction is spontaneous or non-spontaneous, or whether that depends on temperature. Case #1: The reaction is always spontaneous. The diagram below represents a spontaneous reaction deltag 0. Use the diagram to answer the questions below. The following diagram represents an imaginary two step mechanism. Let the red spheres represent element a the green ones element b and the blue ones element c. Chemical equation question with a diagram. Watch the video solution for the question. The figure below represents the spontaneous reaction of h2 shaded spheres with o2 unshaded spheres to.
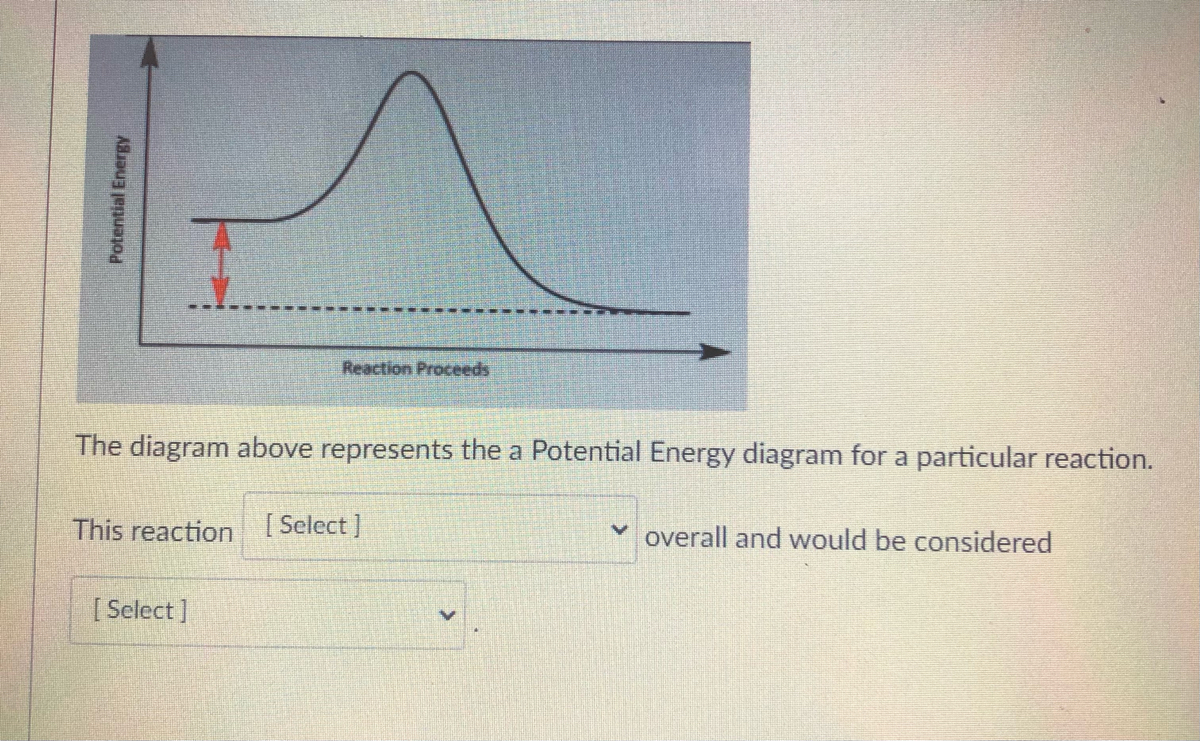
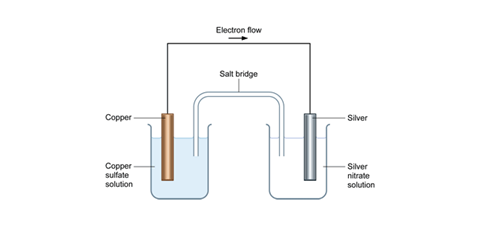
The diagram below represents a spontaneous reaction (δg. Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay"; A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ... j. Based on your answers from c, g and h, determine if the following reaction is spontaneous. Explain. 2Ag(s) + Cu2+(aq) → Cu(s) + 2Ag+(aq) No, the reaction a written above is not spontaneous. Based on the spontaneous reaction written in part c, Cu(s) is more likely to give up electrons and Ag+(aq) is more likely to accept electrons. The reaction SO2(g)+2H2S(g)⇌3S(s)+2H2O(g)SO2(g)+2H2S(g)⇌3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2SO2 from power-plant stack gases. The values below may be helpful when answering questions about the process. Calculate the equilibrium constant KpKpK_p for the reaction at a temperature of 298 KK. The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0).
The diagram above represents the gas-phase reaction of NO 2 (g) to form N 2 O 4 (g) at a certain temperature. Based on the diagram, which of the following best predicts and explains the sign of the entropy change for the reaction, ΔS°rxn ? Spontaneous Reactions. In spontaneity a spontaneous process is an irreversible one that happens without external energy or agents. This process may take place slowly or quickly as it is not related to kinetics of reaction rate. A spontaneous reaction is a kind of reaction that happens naturally in a given set of conditions without any intervention. The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below. Learn this topic by watching Gibbs Free Energy Concept Videos. All Chemistry Practice Problems Gibbs Free Energy Practice Problems. Q. The reaction SO2 (g) + 2H2S (g) ⇌ 3S (s) + 2H2O (g) is the basis of a suggested method for removal of SO2 from power-plant gases. For the reaction below ΔG° = + 33.0 kJ, ΔH° = + 92.2 kJ, and ΔS° = + 198.7 J/K. Estimate ... The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. ... According to the diagram above, ΔG° is positive and the equilibrium composition is rich in reactants.
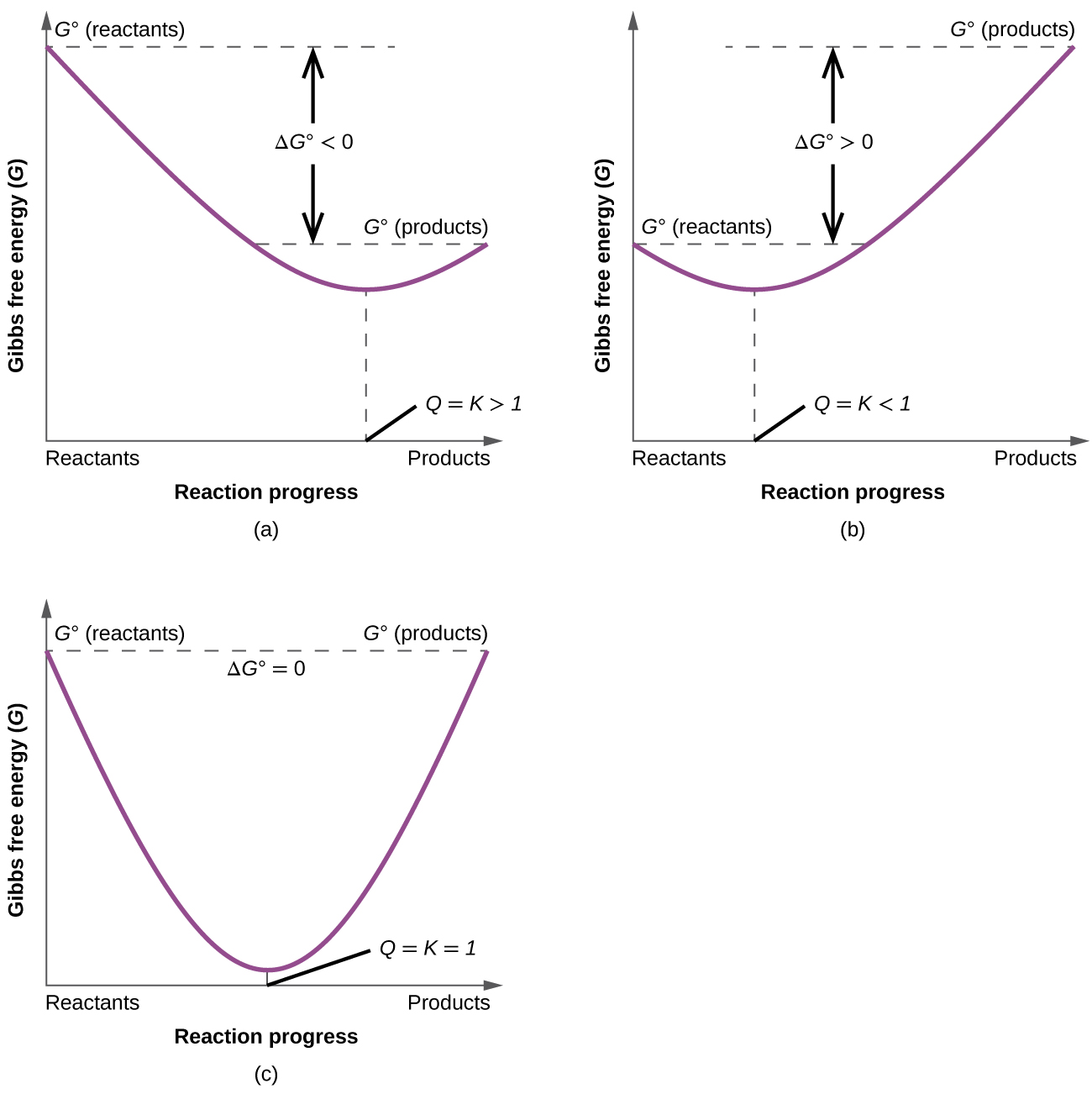
25 Apr 2018 — Problem: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below.a. Is the reaction endo the rmic or ...1 answer · Top answer: a) exo the rmic; b) 25 kJ The diagram below represents a spontaneous reaction (deltaG degree < 0). Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state. The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether {eq}\Delta H, \ \Delta S, \ and \ \Delta G{/eq} for this reaction are positive, negative, or zero. QUESTION 1- Given The Free Energy Diagram Below, Select ALL Answers That Are Correct: ΔG° > 0 ΔG° < 0 ΔG° = 0 K > 1 K < 1 K = 1 K = 0 ΔG (point 3) > 0 ΔG (point 3) < 0 ΔG (point 3) = 0 Q > K (point 1) Q < K (point 1) Q = K (point 1) Forward Reaction Spontaneous (point 2) Reverse Reaction Spontaneous (point 2) Reaction At Equilibrium (point ...

Ligand Binding Free Energy And Kinetics Calculation In 2020 Limongelli 2020 Wires Computational Molecular Science Wiley Online Library
Use Equation \(\ref{Eq5}\), the calculated value of ΔS°, and other data given to calculate ΔG° for the reaction. Use the value of ΔG° to determine whether the reaction is spontaneous as written. Solution. A To calculate ΔG° for the reaction, we need to know ΔH°, ΔS°, and T. We are given ΔH°, and we know that T = 298.15 K.
The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG. E) a positive value of Ecell and a value of zero for ΔG.
5. Consider the reaction below and without reference to any data tables, draw the proper conclusion on which condition A-D best represents the reaction being spontaneous as written. CO 2 (g) + H 2O (g) --> HCOOH (l) ΔH = -150 kJ A. The reaction would be spontaneous at all temperatures. B.
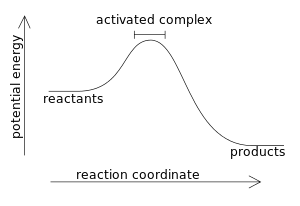
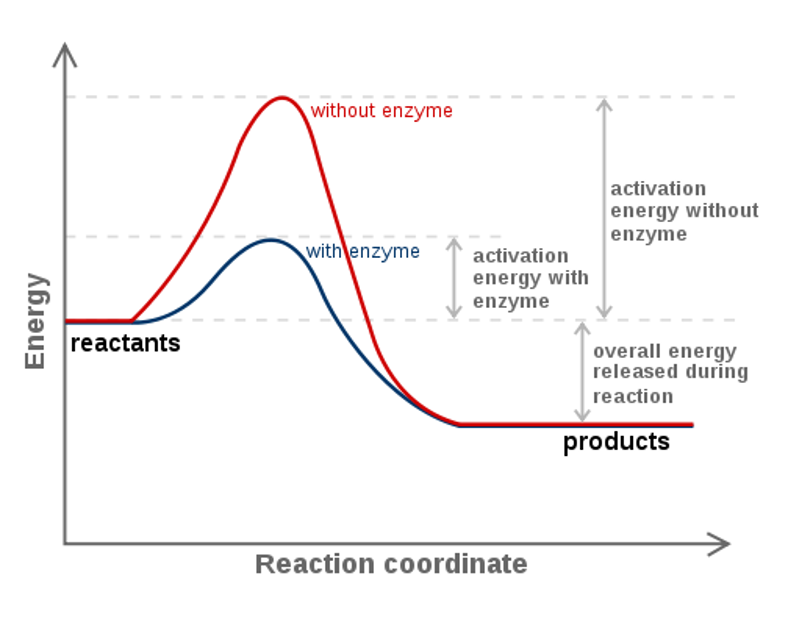
Energy reaction progress which curve represents the catalyzed reaction. Energy diagrams depict the reaction progress versus energy. First as noted the y axis is labeled enthalpy and the x axis is labeled reaction progress then we have the actual energy diagram plot. Identify the key features of the reaction profile below.

Synergistic Effect Of Glutaric Acid And Ammonia Amine Amide On Their Hydrates In The Clustering A Theoretical Study Sciencedirect
14 Dec 2020 — The diagram below represents a spontaneous reaction. Drag the labels to the correct bins. Is the reaction endo the rmic or exo the rmic? The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process.
Which of the following is NOT true for ΔGrxn? A.) If ΔG°rxn > 0, the reaction is spontaneous in the forward direction. B.) If Q = 1, then ΔGrxn = ΔG°rxn.If ΔG°rxn = 0, the reaction is spontaneous in the reverse direction. Chemistry. For a particular reaction, ΔH° is 20.1 kJ/mol and ΔS° is 45.9 J/(mol·K).
Entropy is a mathematically defined property in thermodynamics. It can often help to understand it as a measure of the possible arrangements of the atoms, ions, or molecules in a substance. The symbol for entropy is S, and a change in entropy is shown as "delta" S or ΔS. If the entropy of a system increases, ΔS is positive.

Removal Of Congo Red By Silver Carp Hypophthalmichthys Molitrix Fish Bone Powder Kinetics Equilibrium And Thermodynamic Study
18. Determine the minimum temperature for a reaction with ΔH = 271 kJ and ΔS = 195 J/K to be spontaneous. When ΔG = 0 the reaction is at equilibrium, so solve for T under these conditions. ΔG = ΔH - TΔS = 0 T = ΔH/ΔS = 271 kJ / (0.195 kJ/K) = 1389.74 K 19. Consider the reaction: CO(g) + Cl2(g) → COCl2(g) Calculate ΔGrxn at 25 °C
ΔG represents the change in Gibbs free energy for a chemical system at constant temperature and pressure ΔG = ΔH -TΔS ... We can write an equation to represent this entropy change in the surroundings at constant temperature as shown below: ... For a spontaneous reaction, ΔG for the reaction is negative (ΔG < 0).
Energies Free Full Text Recent Advances In Thermochemical Energy Storage Via Solid Gas Reversible Reactions At High Temperature Html
Question: Label the components of an energy diagram for a spontaneous reaction. Answer Bank reactants activation energy products uncatalyzed reaction catalyzed reaction Reaction progress- This problem has been solved! See the answer See the answer See the answer done loading.
Problem: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right.
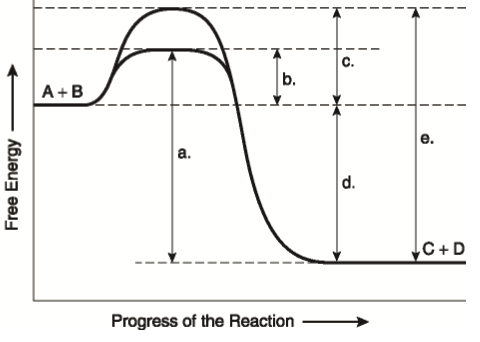
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.
ΔG = ΔH - TΔS. Remember that for a reaction to be feasible, ΔG has to be negative. ΔH could be negative (an exothermic reaction) or positive (an endothermic reaction). Similarly ΔS could be either positive or negative. There are four possible combinations of the signs of ΔH and ΔS. I want to look at those in turn.
Frontiers Green Synthesis Of Ph Responsive Self Assembled Novel Polysaccharide Composite Hydrogel And Its Application In Selective Capture Of Cationic Anionic Dyes Chemistry
C)It is non-spontaneous and produces an electric current. D)It is non-spontaneous and requires an electric current. (1)Which statement describes the redox reaction that occurs when an object is electroplated? Base your answers to questions 2 and 3 on the diagram below which represents the electroplating of a metal fork with Ag(s). A)Ag+ + e ...
The diagram below represents a spontaneous reaction deltag 0. Use the diagram to answer the questions below. The following diagram represents an imaginary two step mechanism. Let the red spheres represent element a the green ones element b and the blue ones element c. Chemical equation question with a diagram. Watch the video solution for the question. The figure below represents the spontaneous reaction of h2 shaded spheres with o2 unshaded spheres to.
These are included in an equation to tell you whether a reaction happens: ΔG = ΔH - TΔS Depending on the combination of signs you have for these two state functions, you'll be able to determine whether a reaction is spontaneous or non-spontaneous, or whether that depends on temperature. Case #1: The reaction is always spontaneous.
chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so Δs will be positive as Δh = Δg tΔs this means that Δh Δg for the other reactions there is a decrease in the number of moles of has so Δs is negative Δh = Δg tΔs this means that Δh Δg

Temperature And Salt Effects Of The Kinetic Reactions Of Substituted 2 Pyridylmethylene 8 Quinolyl Iron Ii Complexes As Antimicrobial Anti Cancer And Antioxidant Agents With Cyanide Ions

The Diagram Below Represents A Spontaneous Reaction Deltag Degree 0 Drag The Labels To The Homeworklib
Optical Trapping Enhancement From High Density Silicon Nanohole And Nanowire Arrays For Efficient Hybrid Organic Inorganic Solar Cells Rsc Advances Rsc Publishing Doi 10 1039 C4ra13536a

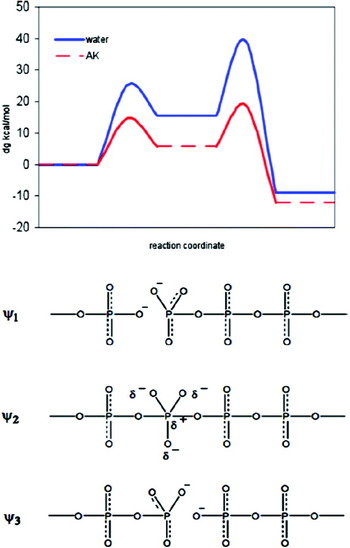








0 Response to "37 the diagram below represents a spontaneous reaction (δg"
Post a Comment