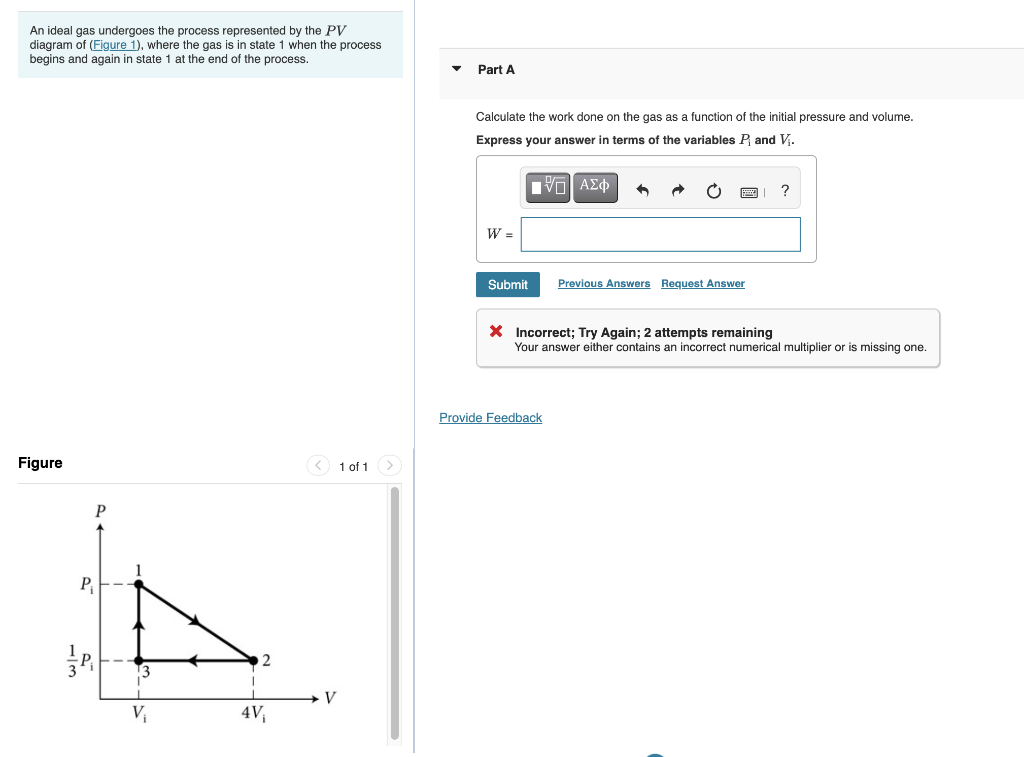
37 a gas undergoes a change of state described by the pvpv diagram shown in the figure below.
As obvious from the given diagram, magnitude of area under the isothermal curve is greater than the same under adiabatic curve, hence $W_{isothermal} > W_{adiabatic}$. 10. In the cyanide extraction process of silver from argentite ore, the oxidising and reducing agents used are. However in various heat engines, gas turbines, and steam power plants the heat is obtained from The Combustion Process - The basic combustion process can be described by the fuel (the hydrocarbon) plus oxydizer (air or oxygen) called the Reactants, which undergo a chemical process...
Multiple Choice Questions Introduction to Geology - Chapter 1. Each chapter will include a few questions designed to test your knowledge of material covered in the chapter and in the Internet-based resources. Your answers are not being recorded.

A gas undergoes a change of state described by the pvpv diagram shown in the figure below.
The figure shows a cycle undergone by 1.41 mol of an ideal monatomic gas. For 1 to 2, what are (a) heat Q, (b) the change in internal energy ΔEint, and A heat engine with 0.227 moles of a monatomic gas undergoes the cyclic procedure shown in the pV diagram on the right. Between stages 3 and 1... Figure 3 shows the phase diagram for water. Using the graph, if you know the pressure and temperature you can determine the phase of water. This is known as the triple point and is described by a single point on a phase diagram. A gas at a temperature below its boiling point is called a vapor. It then undergoes a reversible isothermal expansion until P= 1.10 bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate wfor each step and for the total process. What values for wwould you calculate if the cycle were traversed in the opposite direction?
A gas undergoes a change of state described by the pvpv diagram shown in the figure below.. Transcribed image text: A gas undergoes a change of state described by the p V diagram shown in the figure below. 4.000 m 3.00 2.00 1.00 1.00 2.00 3.00 4.00 Vim 1) Calculate the amount of work done on the gas. (Express your answer to three significant figures.) 1.curve PQ- A reversible isothermal gas expansion process. In this process, the ideal gas in the system absorbs Q amount heat from a heat source at a high temperature T, expands and does work on surroundings. > The state of a gas in a cylinder is represented by the pV diagram shown below. For the circuit shown in the figure, calculate (a) the current in the 2.00-Ω resistor and (b) the potential difference between points a and b, ΔV = Vb - Va. Note: a gas can follow several different paths from state 1 to 2, and each path will have a different area A gas in piston‐cylinder assembly undergoes a polytropic expansion. The initial pressure is 3 bar Two closed systems are under consideration, as shown in schematic. The only heat transfer is...
A gas undergoes a thermodynamic cycle consisting of the followingprocesses:(i) Process 1-2 There are nosignificant changes in KE and PE.(a) Sketch the cycle on a p-V diagram(b) Calculate A gas undergoes a thermodynamic cycle consisting of three processesbeginning at an initial state where... ...pvpv-diagram-shown-figure--1-calculate-amount-work-do-q25825551. By continuing to use Pastebin, you agree to our use of cookies as described in the Cookies Policy. OK, I Understand. So, the climate change is an effective and long-term change in weather rate occurring for a particular area. The weather rate includes temperature, rainfall, and wind condition. Climate change is one of the most dangerous phenomena in the life of the planet. Substances can change state, usually when they are heated or cooled. For example, liquid water turns into steam when it is Simple diagrams of particles in a solid, liquid and a gas are shown like this Become able to move quickly in all directions. Evaporation happens below the boiling point of a liquid.
(Figure) shows a gas confined by a membrane to one side of a two-compartment, thermally insulated container. When the membrane is punctured There is no change in the internal energy of an ideal gas undergoing an isothermal process since the internal energy depends only on the temperature. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly In such a gas, all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature. Gas properties are described in terms of state variables. This change is known as change of state. The graph in Figure 11 shows the changes in temperature as thermal energy is gradually added to a container During freezing, the temperature of a substance remains constant while the particles in the liquid form a crystalline solid. Because particles in a liquid... The document shows that emissions of greenhouse gases from human activities are responsible for approximately 1.1°C of warming between Extreme sea level events that previously occurred once in 100 years could happen every year by the end of this century. The report also indicates that further...

An Ideal Gas Of N Moles Undergoes The Reversible Process Ab Shown In The Pv Diagram Below The Temperature T Of The Gas Is The Same At Points A And B Determine
ShowMore Show More. While companies have a huge role to play in driving climate change, says Faria, the barrier is the "absolute tension" between short-term profitability and the urgent need A fifth of global industrial greenhouse gas emissions are backed by public investment, according to the report.

Figure Shows The P V Diagram Of An Ideal Gas Undergoing A Change Of State From A To B Four Different Parts I Ii Iii And Iv As Shown In The Figure May
The change in the direction of light from medium to medium is because of the change in speed it undergoes. The relation that is not too difficult to derive is called the "Snell's Law of Refraction" as shown below Solution: As the figure shows, the angle of incidence is not 40.0° degrees.
They said Rowling will be shown in archival footage but will not make any new appearances in the special. There will also be a first look of the special during the premiere of quiz competition show Harry Potter: Hogwarts Tournament of Houses which airs Sunday, November 28 on TBS and Cartoon...
Research shows that changes in climate create warmer, drier conditions. Increased drought, and a longer fire season are boosting these increases in wildfire risk. Changes in climate add to these factors and are expected to continue to increase the area affected by wildfires in the United States.
A state diagram is a type of diagram used in computer science and related fields to describe the behavior of systems. State diagrams require that the system described is composed of a finite number of states; sometimes, this is indeed the case, while at other times this is a reasonable abstraction.

A Monatomic Ideal Gas Is Taken Around The Cycle In Figure 1 In The Direction Shown The Path For The Process C To A Is A Straight Line In The Pv Diagram A
During this change from A to B. P A 20 15 10 5 B 0 2 4 6 8 10. We're in the know. This site is using cookies under cookie policy. You can specify conditions of storing and accessing cookies in your browser.
The diagram shows changes of state between solid, liquid, and gas. How is energy related to the change of state represented by the model? Atoms gain energy as a solid changes to a liquid. If atoms energy during a change of state, they are pulled together by attractive forces and become more...

A Heat Engine With 0 227 Moles Of A Monatomic Gas Undergoes The Cyclic Procedure Shown In Homeworklib
In the $p V$ diagram shown in Figure $15.38,85. \mathrm{~J}$ of work was done by 0.0650 mole of ideal gas during an adiabatic process. (a) How Therefore the internal energy of the gas decreases by the work that is being done again, 85 jewels. And finally we know that for part C, we can use the...
European natural gas prices have soared so high that hundreds of millions of people could be facing cold homes or inflated energy bills over winter. There's also fears of a knock-on impact as carbon dioxide used in food production — a byproduct of fertilizer made with natural gas — also gets more...
* Land Use, Land-Use Change, and Forestry in the United States is a net sink and removes approximately 12 percent of these greenhouse gas emissions, this net sink is not shown in the above diagram. All emission estimates from the Inventory of U.S. Greenhouse Gas Emissions and Sinks...
A table I made (below) of gas densities at these conditions show values for density about 1-5x greater than what the density they would be at ambient Clearly, these theoretical conditions are difficult to obtain in the case of real gases because (1) real molecules do have some size or volume of their...
Changes in greenhouse gas concentrations, dominated by CO2, caused a large warming contribution. Some of this has been offset by the net Figure 3.4: Human-induced drivers of climate change have been much larger than natural drivers over the last century. The strength of these drivers, which are...

Figure Shows The P V Diagram Of An Ideal Gas Undergoing A Change Of State From A To B Four Different Process I Ii Iii Iv As Shown In Figure May Lead To
It then undergoes a reversible isothermal expansion until P= 1.10 bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate wfor each step and for the total process. What values for wwould you calculate if the cycle were traversed in the opposite direction?
Figure 3 shows the phase diagram for water. Using the graph, if you know the pressure and temperature you can determine the phase of water. This is known as the triple point and is described by a single point on a phase diagram. A gas at a temperature below its boiling point is called a vapor.
The figure shows a cycle undergone by 1.41 mol of an ideal monatomic gas. For 1 to 2, what are (a) heat Q, (b) the change in internal energy ΔEint, and A heat engine with 0.227 moles of a monatomic gas undergoes the cyclic procedure shown in the pV diagram on the right. Between stages 3 and 1...
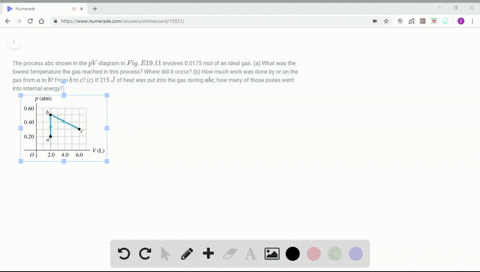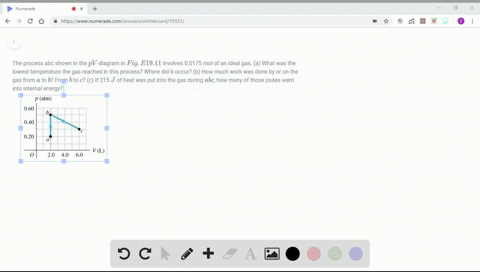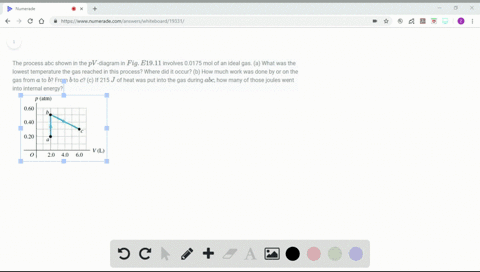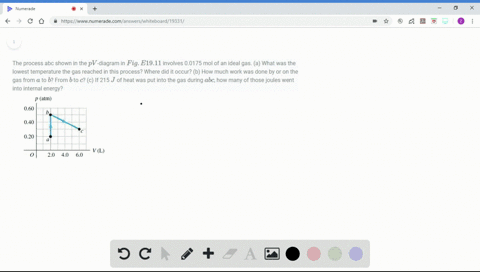
Solved Figure P19 43 Shows A Pv Diagram For 0 0040 Mol Of Ideal H 2 Gas The Temperature Of The Gas Does Not Change During Segment Bc A What Volume Does This Gas Occupy At Point

Figure Shows The P V Diagram Of An Ideal Gas Undergoing A Change Of State From A To B Four Different Process I Ii Iii Iv As Shown In Figure May Lead To

A Heat Engine Using A Monatomic Gas Follows The Cycle Shown In The Pv Diagram Below The Gas Starts Out At Point 1 With A Volume Of 233 Cm 3 A Pressure 147

Pdf Closed State Inactivation Involving An Internal Gate In Kv4 1 Channels Modulates Pore Blockade By Intracellular Quaternary Ammonium Ions

An Ideal Gas Undergoes Cyclic Process Abcda As Shown In Given Pv Diagram The Amount Of Work Done By The Gas Is

Ps2 Sp11 Key Department Of Chemistry Nbsp Sbquo P2 2 The Temperature Of 2 50 Moles Of An Ideal Gas Increases From 13 5 Sbquo Deg C To 55 1 Sbquo Deg C As The Gas Is Microsoft Word Pdf
Nanomaterials Free Full Text Tuning Of Ag Nanoparticle Properties In Cellulose Nanocrystals Ag Nanoparticle Hybrid Suspensions By H2o2 Redox Post Treatment The Role Of The H2o2 Agnp Ratio Html
















0 Response to "37 a gas undergoes a change of state described by the pvpv diagram shown in the figure below."
Post a Comment