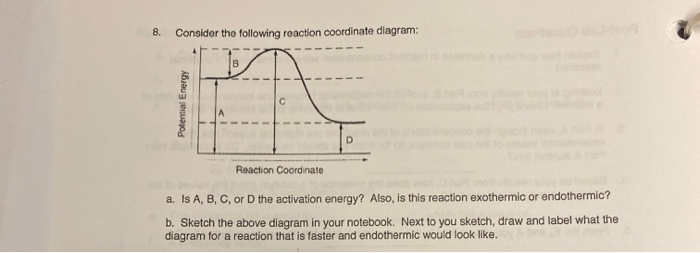
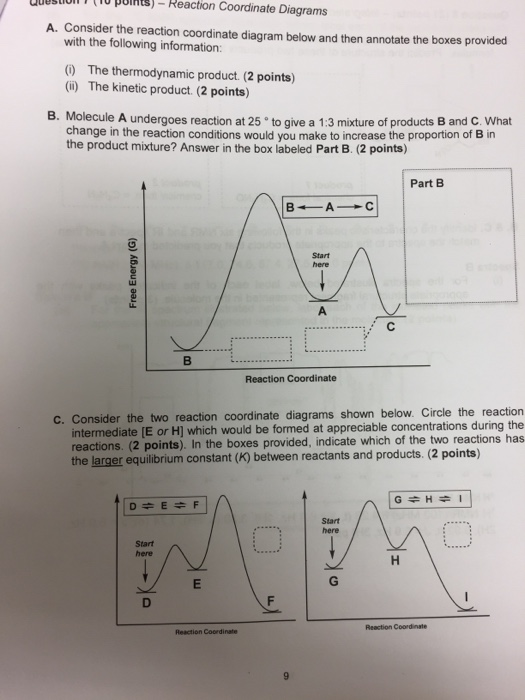
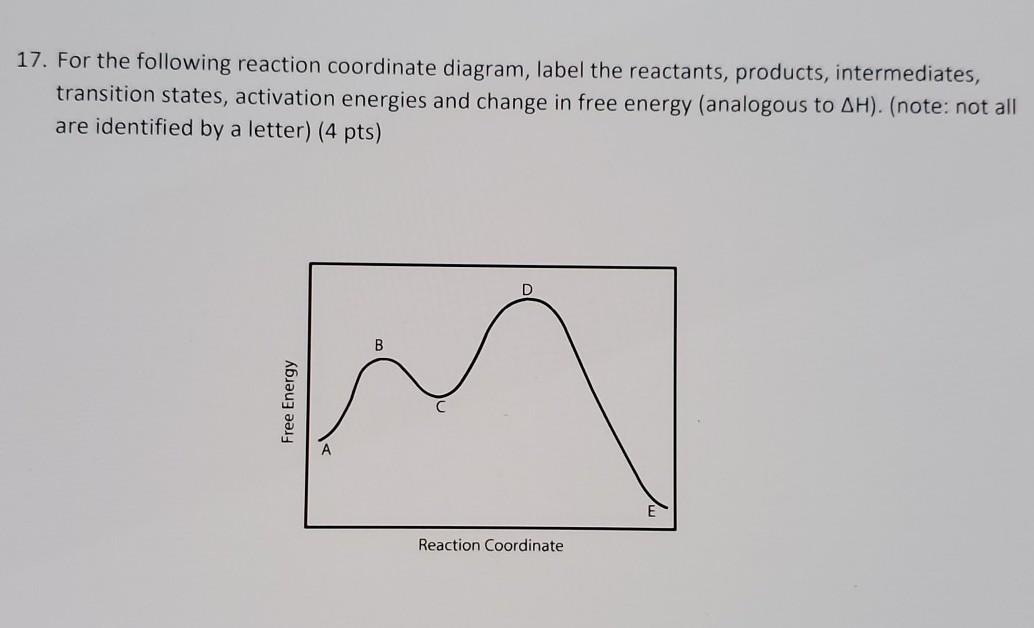
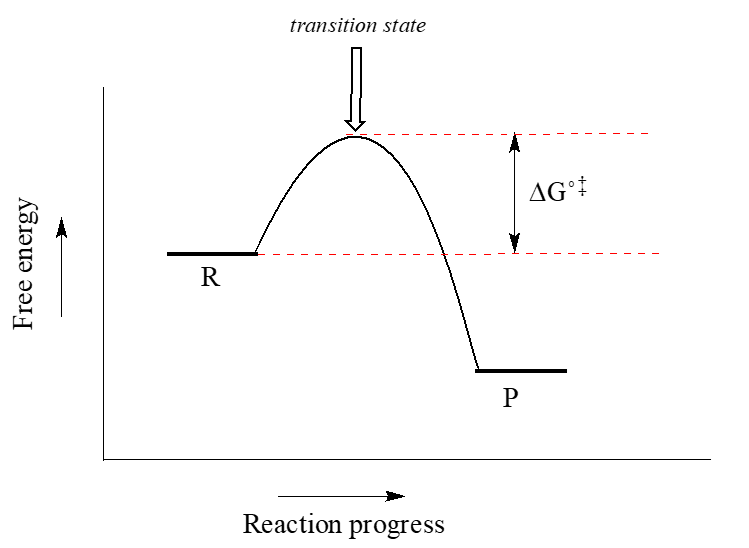
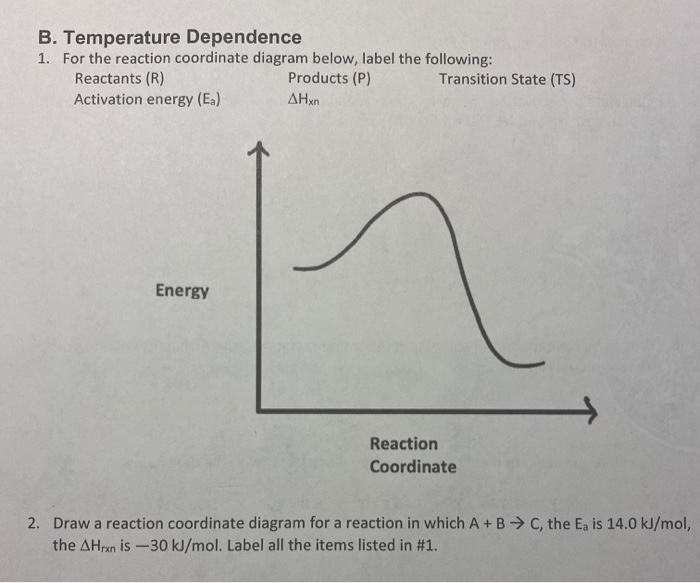
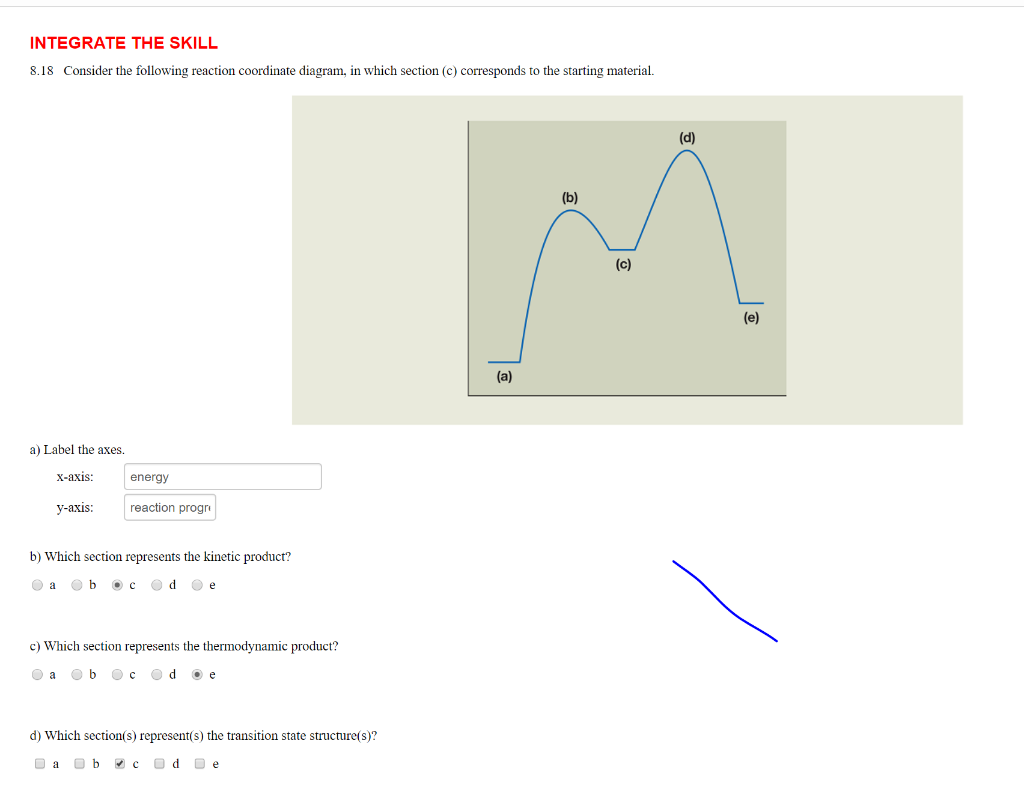
37 label the following reaction coordinate diagram.
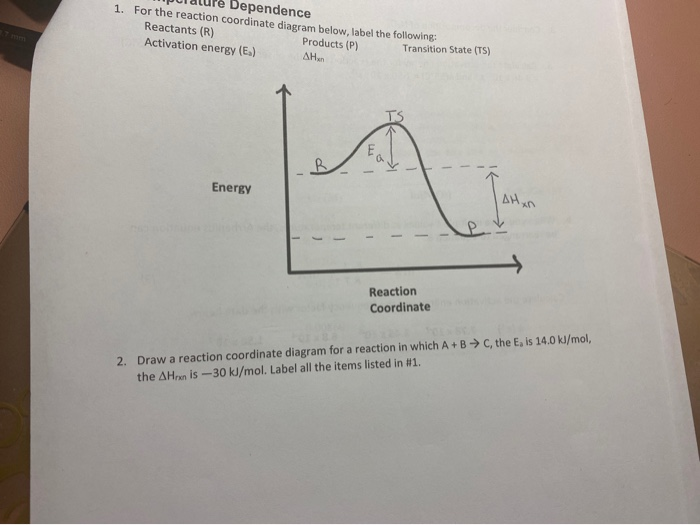
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. Answer to Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region. If playback doesn't begin shortly, try restarting your device.
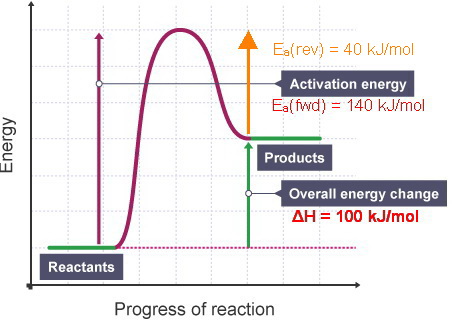
The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.

Label the following reaction coordinate diagram.
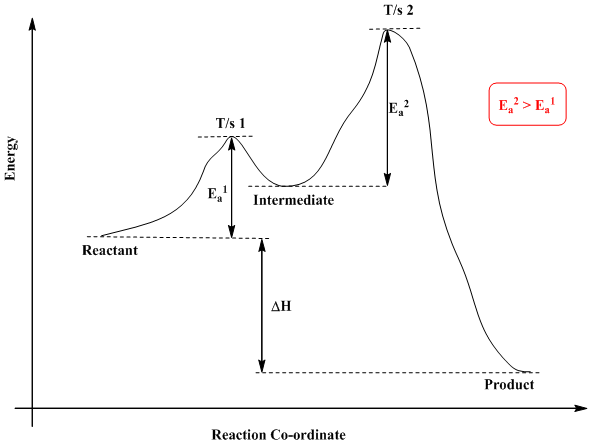
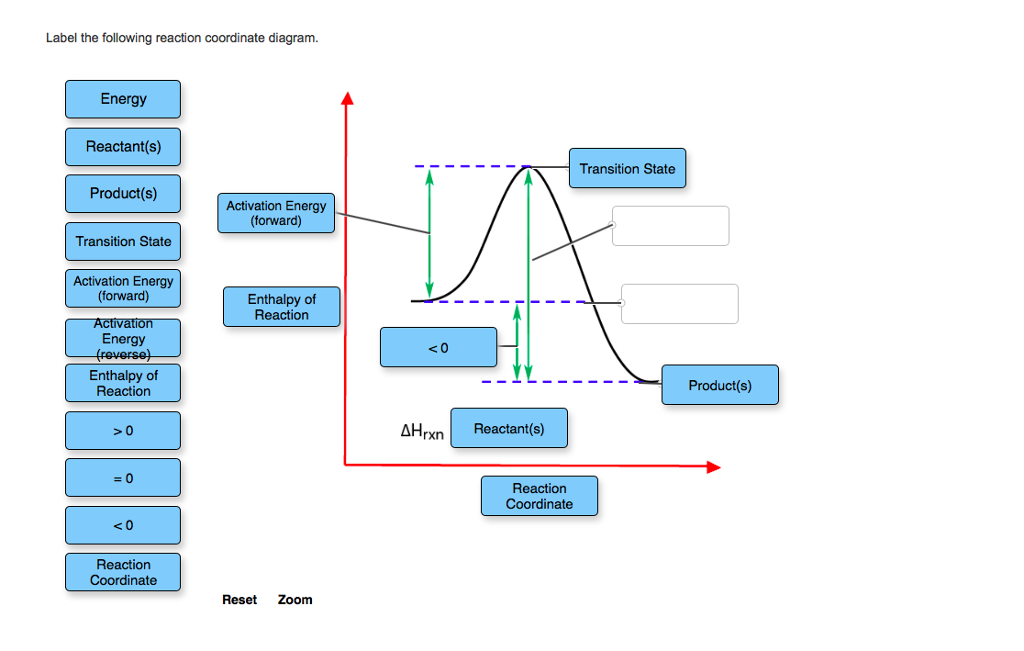
Chemistry questions and answers. Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom. Label them A through...on the diagram. (b) Label the transitions states on the diagram. (c) Which is the fastest step in the reaction? (d) Are the reactants or products more stable? (e) Which is the most stable intermediate? (1) Question: 6. Given the following reaction coordinate diagram, answer these questions: (a) How many intermediates are ... A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state functions If ΔSo is small, compared to ΔHo, then ΔGo o ΔE = q + w! ΔH = q p!
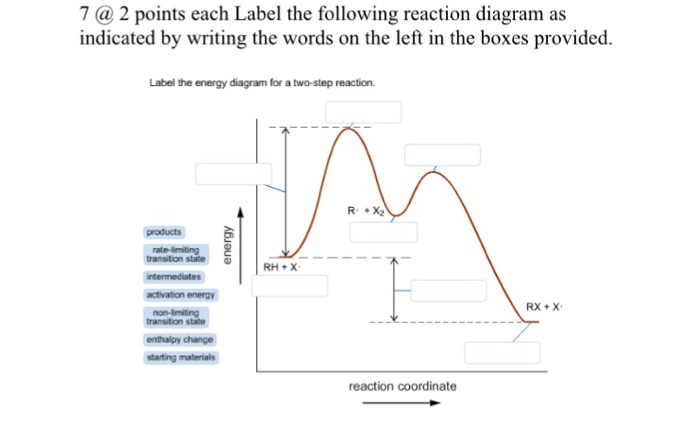
Label the following reaction coordinate diagram.. Label the following reaction coordinate diagram. enthalpy of reaction, reactant (s), reaction coordinate, activation energy (reverse), transition state, < 0, > 0, activation energy (forward), = 0, product (s), energy. Our mission is to help you succeed in your Chemistry class. "Clutch really helped me by reinforcing the things I learned in ... Chapter 7. Label the different energies on the following energy diagram. On this graph, the x -axis is the reaction coordinate, while the y -axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction. Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. Problem Details. Label the following reaction coordinate diagram by matching between letters and numbers: All Chemistry Practice Problems Energy Diagram Practice Problems. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W...
A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state functions If ΔSo is small, compared to ΔHo, then ΔGo o ΔE = q + w! ΔH = q p! Label them A through...on the diagram. (b) Label the transitions states on the diagram. (c) Which is the fastest step in the reaction? (d) Are the reactants or products more stable? (e) Which is the most stable intermediate? (1) Question: 6. Given the following reaction coordinate diagram, answer these questions: (a) How many intermediates are ... Chemistry questions and answers. Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom.

Homework Saved Label The Following Reaction Coordinate Diagram Activation Energy Poversa 0 A Enthalpy Of Reaction Homeworklib
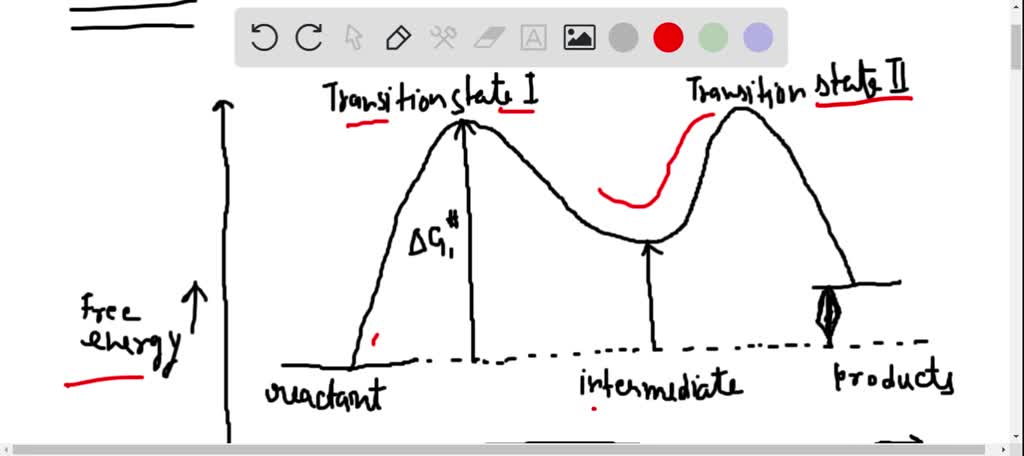
Solved Draw A Fully Labeled Reaction Coordinate Diagram That Meets The Following Criteria 1 A 2 Step Process 2 Each Step Is Exergonic 3 The Second Step Is Slower Than The First

2 Given The Reaction Coordinate Diagram Below Answer The Following Questions Free Energy Progress Of The Homeworklib
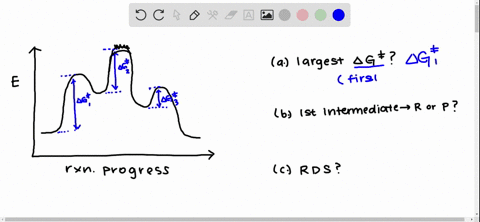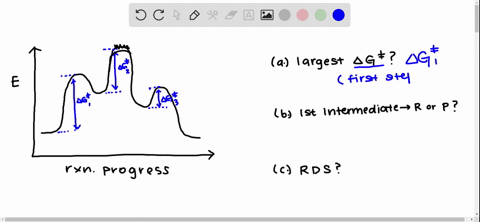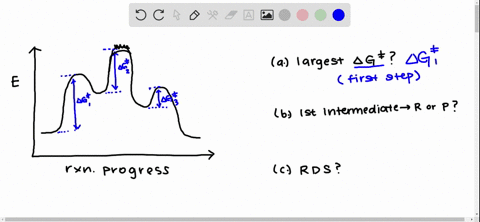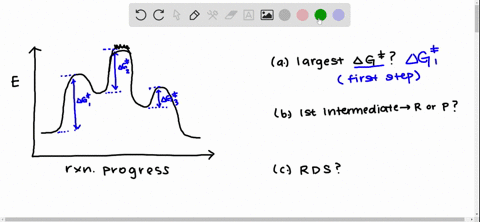
Solved Draw A Reaction Coordinate Diagram For A Two Step Reaction In Which The First Step Is Endergonic The Second Step Is Exergonic And The Overall Reaction Is Endergonic Label The Reactants Products Intermediates

Solved 65 A Draw The Reaction Coordinate Diagram For An Exothermic Reaction Label The Following On The Diagrams Reactants And Products Ah Activation Energy And Transition State B Show On Your Reaction Coordinate
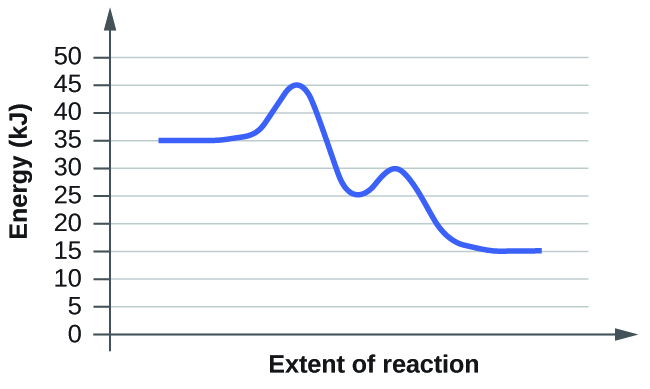

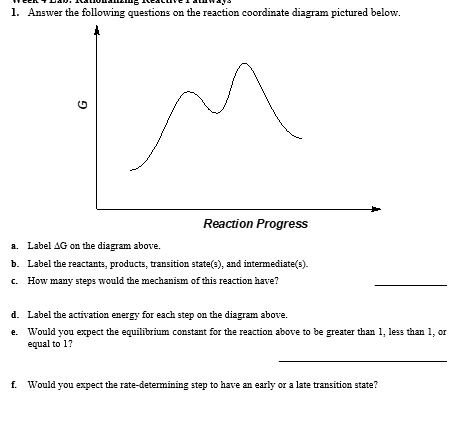
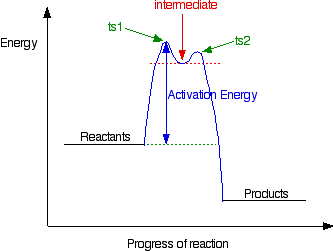















0 Response to "37 label the following reaction coordinate diagram."
Post a Comment