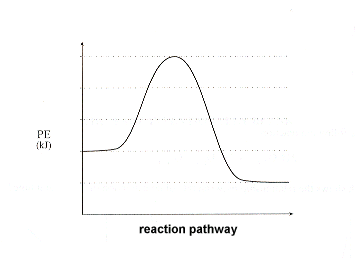
38 energy diagram for exothermic reaction
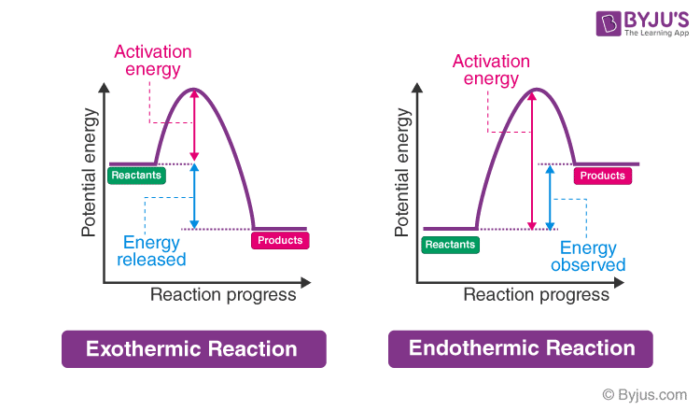
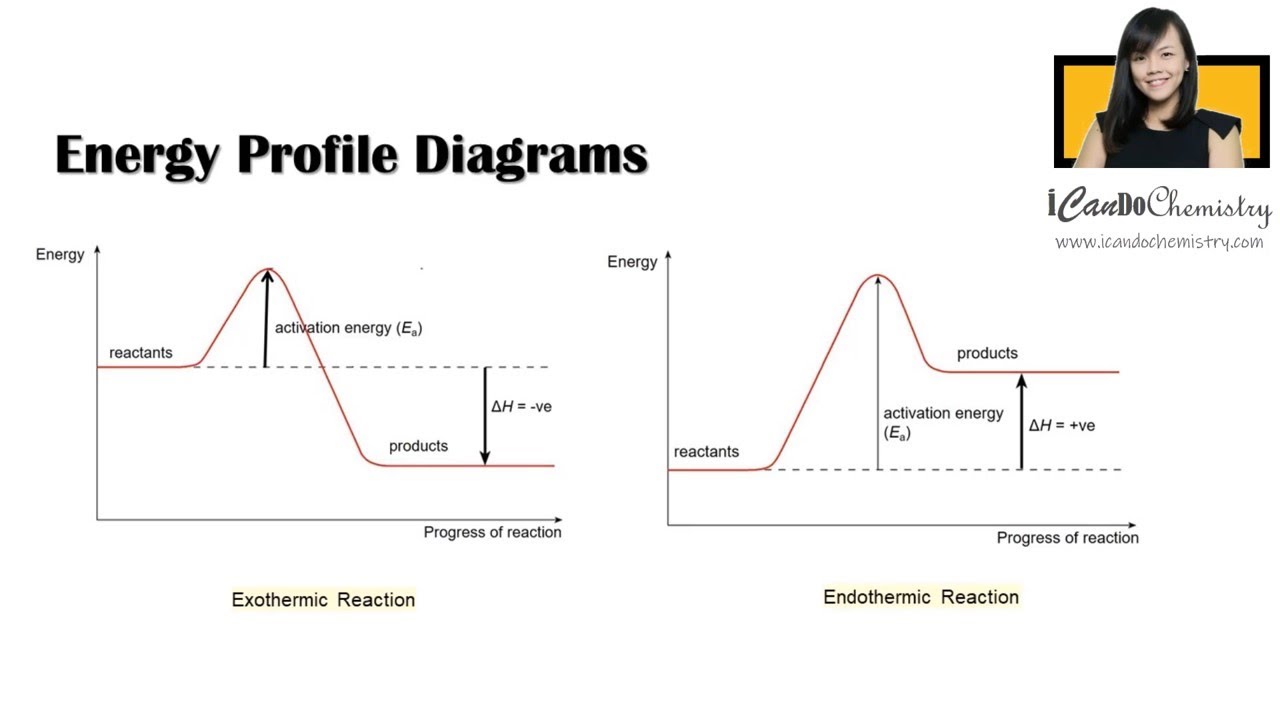
1590s, "force of expression," from French énergie (16c.), from Late Latin energia, from Greek energeia "activity, action, operation," from energos "active, working," from en "at" (see en- (2)) + -ergos "that works," from ergon "work, that which is wrought; business; action" (from PIE root *werg- "to do"). Used by Aristotle with a sense of "actuality, reality, existence" (opposed to "potential") but this was misunderstood in Late Latin and afterward as "force of expression," as the power which calls up realistic mental pictures. Broader meaning of "power" in English is first recorded 1660s. Scientific use is from 1807. Energy crisis first attested 1970. From the given energy diagram we conclude that this is an exothermic reaction diagram. Thus, the enthalpy of reaction is, -20 kJ. The enthalpy of reaction is the amount of heat that is required or to be released in order to convert the reactants to the products. this can be calculated through the equation, δh = h (products) - h (reactants)
"action in resistance or response to another action or power," 1640s, from re- "back, again, anew" + action (q.v.). Modeled on French réaction, older Italian reattione, from Medieval Latin reactionem (nominative reactio), a noun of action formed in Late Latin from the past-participle stem of Latin reagere "react," from re- "back" + agere "to do, perform." Originally a word in physics and dynamics. In chemistry, "mutual or reciprocal action of chemical agents upon each other," by 1836. The general sense of "action or feeling in response" (to a statement, event, etc.) is recorded from 1914. Reaction time, "time elapsing between the action of an external stimulus and the giving of a signal in reply," attested by 1874.
Energy diagram for exothermic reaction
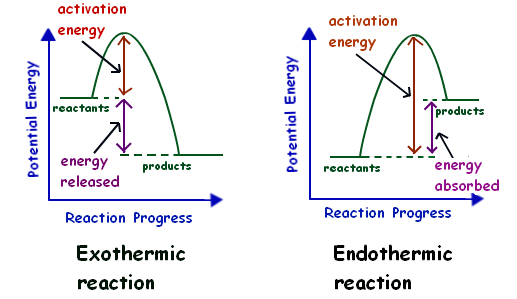
In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants. What is an energy diagram? Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer-- Representing endothermic and exothermic processes using energy diagrams. Oct 08, 2018 · In chemistry, an exothermic reaction refers to a chemical reaction that results in the release of some quantity of energy, normally in the form of light or heat.The opposite of an exothermic reaction is an endothermic reaction, one that takes in heat from the surrounding environment. The characteristics of an exothermic reaction can be expressed with the general chemical equation: …
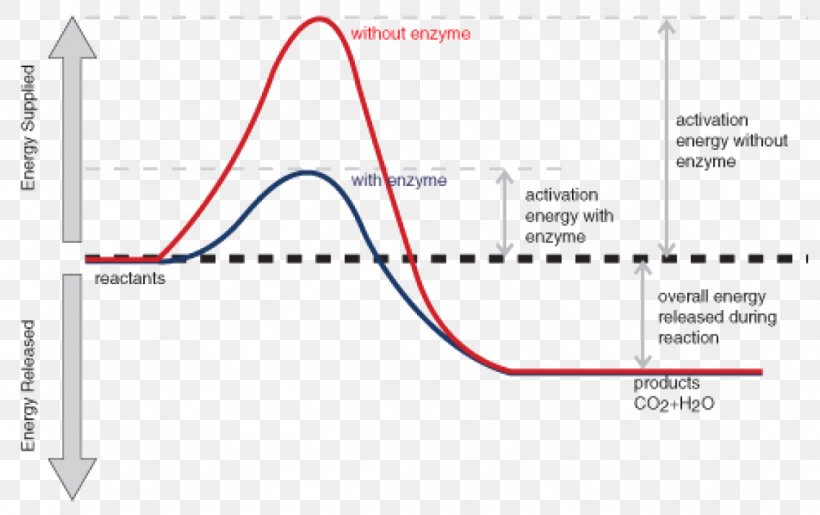
Energy diagram for exothermic reaction. *Originally posted in* [*/r/DestinyLore*](https://www.reddit.com/r/DestinyLore) # It's Hive, so I'm sure it's going to be strange. But at its core, their "magic" is still just science[.](https://bungie.net/common/destiny_content/grimoire/hr_images/603040_865612de633ac3e8a3c8c793f6fd0122.jpg) – [Ana Bray](https://www.ishtar-collective.net/transcripts/adventure-deathly-tremors) I’ve wanted to make this post for some time now and have been considering the science of Hive Magic since Stasis was r... A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ... A heat absorption reaction is endothermic. Its enthalpy will be positive, and its surroundings will cool down. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in heat the device emits, the atmosphere may rise in temperature. ENERGY DIAGRAM. • ΔH = HPRODUCTS. – H. REACTANTS. • IF ENERGY OF REACTANTS IS HIGHER. THAN PRODUCTS. • EXOTHERMIC REACTION. • ΔH = NEGATIVE. • IF ENERGY OF ...11 pages
The change in energy for the above reaction is represented in the energy level diagram given below. SN2 is a single-step reaction, so the diagram only shows one curve. The lower energy of the products CH 3 OH and Br - compared to the reactants CH 3 Br and OH - suggests that the reaction is exothermic and that the products are more stable. The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. The forward reaction is endothermic. The activation energy is the same for the forward and reverse reactions. C. The forward reaction is exothermic. The activation energy is different for the forward and reverse reactions. D. The forward reaction is exothermic. The activation energy is the same for the forward and reverse reactions. 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881).
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial. The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. Energy level diagrams for exothermic reactions. In an exothermic reaction, reactants have more energy than the products. The difference between these two energy ...8 pages 12+ Reaction Energy Diagram. 1 revisit lewis acid/base reactions. Energy level diagrams for exothermic and endothermic reactions showing the activation energy (ea) 'hump' which must be overcome before a chemical reaction can take place. N these are called reaction intermediates or simply. This first video takes you through all the basic parts ...
Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ...
Feb 26, 2015 · 1 answerYou can start with a generic potential energy diagram for an exothermic reaction. http://academics.tctc.edu/science/CHM/CHM/ ...
I've been having trouble with this particular set of questions, I can't tell if I just don't grasp the concepts, or if I actually do know the answers and just can't put it together. 34. Assume that the following reaction is a single step reaction in which a C−Br bond is broken as the C−I bond is formed. The heat of reaction is +38 kJ/mol. I−(aq) + CH3Br(aq) + 38 kJ → CH3I(aq) + Br−(aq)CBrHHHCIHHH a. With reference to collision theory, describe the general process that takes place as this reac...
Chemical Reaction Formula Examples: 1. Methane (CH 4) and oxygen (O 2) react to produce carbon dioxide (CO 2) and water (H 2 O).. CH 4 + 2O 2 → CO 2 + 2H 2 O. CH4 and O2 are the reactants, while CO 2 and H 2 O are the products.. 2. Sodium metal (Na) reacts with water (H 2 O) to form sodium hydroxide (NaOH) and hydrogen gas (H 2).. 2Na + 2H 2 O → 2NaOH + H 2
Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
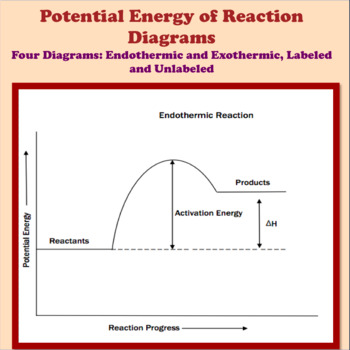
The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex.
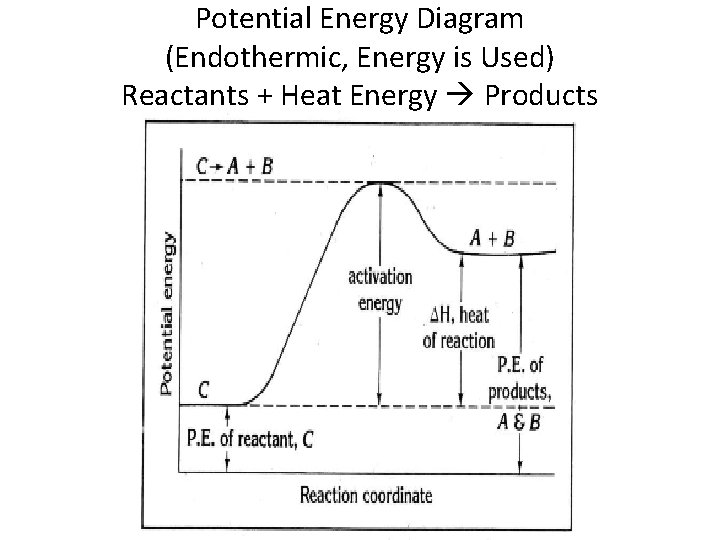
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. The products (C) have higher potential energy than the reactants (A). So, it must have absorbed heat over the reaction to increase its potential energy. When a reaction absorbs heat, it is known as endothermic.
Disclaimer: I'm not interested in debating the science of climate change. If you don't believe in climate change please argue it elsewhere. Thanks! I have an idea for combating climate change, but it involves multiple engineering disciplines which puts most of the feasibility assessment of my idea outside of my expertise. I know myself well enough to know that I'm not cut-out to be an entrepreneur or leader of any sort. My hope is that posting the idea here might inspire some of my fellow engin...
Label The Energy Diagrams Below And Complete The Chegg Com. Draw An Enthalpy Diagram For A General Exothermic Reaction Label The Axis Reactants Products And Del. Write A Balanced Equation And Draw An Enth Clutch Prep. Topic 5 1 Exothermic And Endothermic Reactions Heat And.
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
Endothermic Reaction Energy Diagrams: Endothermic reactions gain energy/heat so when drawing the energy diagram, you want the reactants on the graph to be lower ...
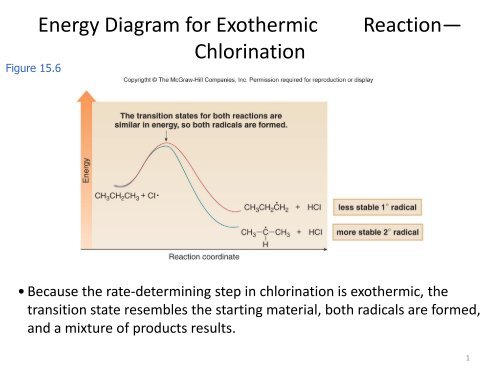
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
An exothermic one releases heat to the surroundings. Both these reaction types cause energy level differences and therefore differences in enthalpy. All you need to remember for the purpose of this calculator is: If the reaction is endothermic, the change in enthalpy is positive, as heat is gained (absorbed from the surroundings).
Exothermic Reactions. A chemical reaction that releases energy (as heat) is called an exothermic reaction.This type of reaction can be represented by a general chemical equation: Reactants → Products + Heat In addition to methane burning, another example of an exothermic reaction is chlorine combining with sodium to form table salt.
Headache, woke up later than usual. Only Chem P1 tips today. To those who are currently taking their papers, good luck with them. ​ Second last day, time sure fly fast. Wash up, have a good breakfast, drink some water, whatever it takes to not burn out. Go run an hour before the exam if you wish to, just don't be late. If you forgot your calculator, either borrow from others or learn quick maths. ​ Papers today: \- (1153/1151/1152)/01 Chinese/Malay/Tamil B P1 08:00 - ...
I'm tutoring someone who is struggling with topics from content category 5E. I already recommended Leah4Sci and they are already using Jack Westin. What other resources did you like for learning these topics besides simply reading a review book? ​ ***Content Category 5E: Principles of chemical thermodynamics and kinetics*** **Enzymes (BC, BIO)** * [Classification by reaction type](https://jackwestin.com/resources/mcat-content/enzymes/classification-by-reaction-type) * [Mechanism]...
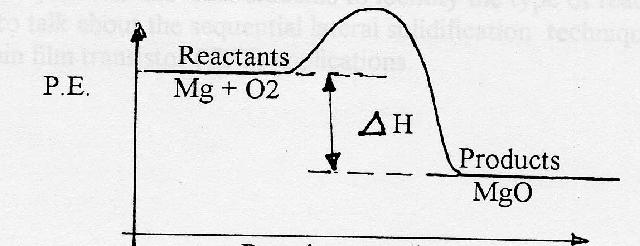
Hey chemhelp, For chemistry I am doing a Report on Reaction Rates. I am almost complete but need help with the last two things. First - finding the ∆H for the following: 2HCl(aq) + Mg(s) -> MgCl(aq) + H2 = -∆H ? Even if you can just help me with the formula, it was 3 cm of Mg ribbon and 5 differ mol/L of HCl (2.0, 1.5, 1.0, 0.5, 0.0) Secondly - I have created a Energy Diagram (exothermic) I need to explain using tentative language why this is related to the experiment.
During an exothermic reaction bonds break and new bonds form and protons and electrons go from a structure of higher potential energy to lower potential energy. What is potential energy stored in? Potential energy is technically stored within matter, though a force must be applied to an object in order for it to store potential energy.
Potential energy diagram worksheet answers 1. δh pep per. The overall difference in potential energy between the products and the reactants. Is the reaction in 6 exothermic or endothermic. Every chemical reaction will either absorb or release energy. For a given reaction the.
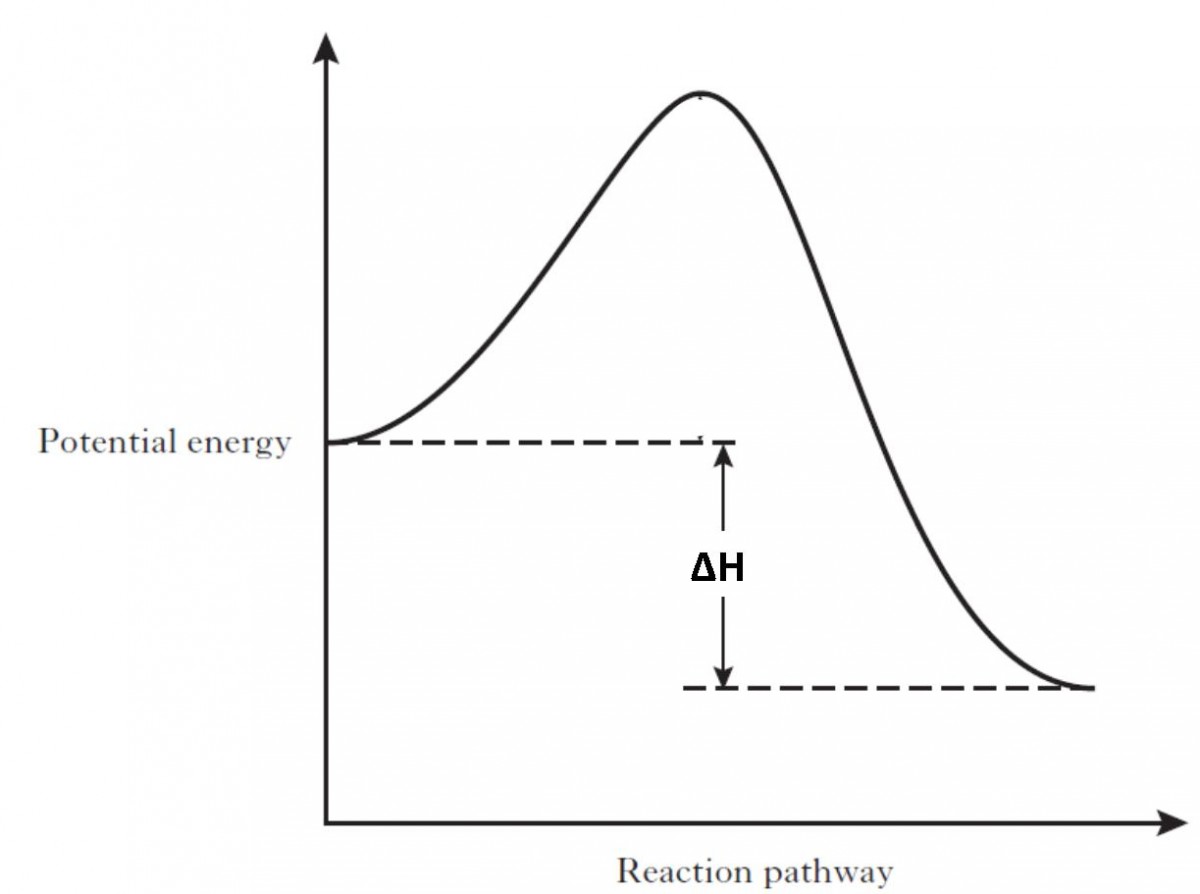
Energy level diagram for an Exothermic change. Thermal energy is released by the System into the Surroundings. This results in a lower Enthalpy value of the final state with respect to the initial ...
Activation energy is the minimum energy required to start a chemical reaction. The colliding particles must possess at least this amount of energy for a reaction to take place. Most of the reactions are exothermic but only a few are totally spontaneous and begin without help at normal temperatures (e.g. sodium or potassium reacting with water).
I am confused on whether in a reaction coordinate diagram, does the difference between the reactants and products equal deltaH or deltaG I have seen graphs that demonstrate both. If some could explain that would be great!   for reference this khan article states it as deltaH: https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermochemistry/a/endothermic-vs-exothermic-reactions   This khan article states it as deltaG: https://www.khanacademy.org/science/biolog...
1874, in chemistry, "relating to a liberation of heat," modeled on French exothermique (1869, Berthelot); see exo- + thermal.
1. If 6.0 grams of NaCl is dissolved in enough water to make a 300.0-ml solution, calculate the w/v percentage of NaCl. 2. What is the molarity of a solution made from 15.5 grams of NaCl dissolved in enough water to make 1.50 liters of solution? 3. How many grams of HNO3 are there in 75.0 ml of 1.25 M HNO3 solution? 4. What is the concentration of a solution made from 23.5 ml of 8.95 M HCl diluted to 95.0 ml? 5. Which solution will have the lowest freezing point: 1 M NaCl, 1 M M...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
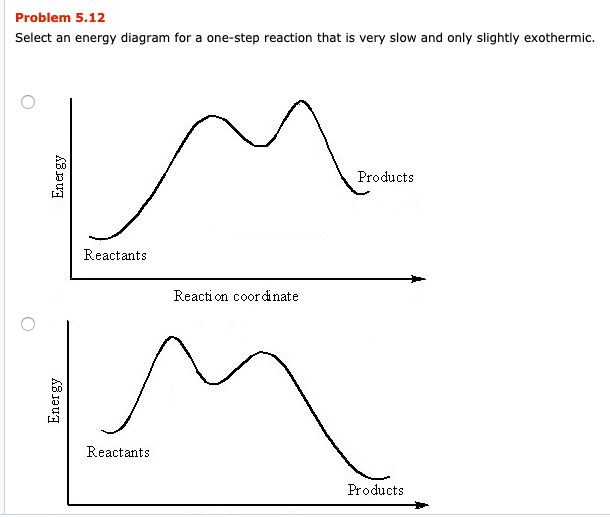
43 Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? (1) The activation energy is 10. kJ and the reaction is endothermic. (2) The activation energy is 10. kJ and the reaction is exothermic.
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below.
1550s, possibly an alteration of tip for tap "blow for blow," from tip (v.3) "tap" + tap "touch lightly." Perhaps influenced by tit (n.2).
Jul 9, 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Apr 09, 2018 · 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
In an exothermic reaction, the change in enthalpy is always negative and energy is being released. When looking at an energy diagram for an exothermic reaction, you will see that the reactants are at a higher energy level while the products are at a visibly lower energy level.
A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. Shows whether a reaction is exothermic. Figure shows the energy level diagram for the reaction between methane and oxygen.
HELP ASAP I WILL GIVE BRAINLIST The potential energy diagram for a reaction starts at 380 kJ and ends at 100 kJ. What type of reaction does the diagram best represent? Exothermic reaction Endothermic reaction Reaction between two solids
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation.
​ It is strongly recommended that you start a book "crazy science" to increase your knowledge. There are PDF arc melting in plasma arc cutting plasma cutting with different working gases can cut all kinds of metals difficult to cut by oxygen, especially for colored metals (stainless steel, aluminum, copper, titanium, nickel). Its main advantages are that When cutting the metal with small thickness, the plasma cutting speed is fast, especially when cutting ordi...
Applications of exothermic and endothermic reactions in everyday life Application of exothermic and endothermic reactions: The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries. Instant cold packs have separate compartments of water and solid ammonium nitrate placed in a plastic […]
The thermodynamic free energy is a concept useful in the thermodynamics of chemical or thermal processes in engineering and science. The change in the free energy is the maximum amount of work that a thermodynamic system can perform in a process at constant temperature, and its sign indicates whether a process is thermodynamically favorable or forbidden.




















0 Response to "38 energy diagram for exothermic reaction"
Post a Comment