38 energy level diagram hydrogen
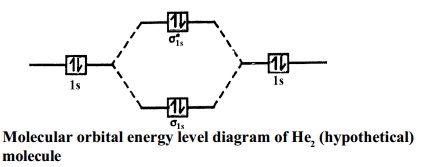
Since Bromine is more electronegative than Hydrogen, the p is at a lower energy level than 1s of hydrogen. The overlap results in sigma bonding and sigma-antibonding orbitals. Conclusion. In this article, we read about the Lewis Structure, Hybridization, Polarity, Molecular Geometry, and MO Diagram of Hydrogen Bromide molecule. Hydrogen is the only element on the left side of the BOLD "staircase" boundary that is a nonmetal ; Elements in same Period have same # of energy levels or shells ; Elements in same Representative Group have same # of valence electrons, except helium in Goup 18.
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time; The transition state is a stage during the reaction at which chemical bonds are partially broken and formed
Energy level diagram hydrogen
an energy level diagram. • The intervals between the energy level becomes smaller and smaller towards the top. ... Draw the energy levels in a hydrogen atom. ii. Show the electron transitions which produce the above lines. iii. Give the name of this particular series of lines. Worked example: Drawing energy level diagrams of the combustion of methane. Answer. Step 1: The chemical equation for the complete combustion of methane is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products. The S 2p 3/2 energy level has a peak at 161.15 eV, and the S 2p 3/2 energy level has a peak at 162.35 eV (Fig. 3d). It is noteworthy that the XPS peaks of Zn, Cd and S of ZCNA-5 move towards lower binding energies compared with ZnCdS, which indicates electron transfer from NiAl LDH to ZnCdS.
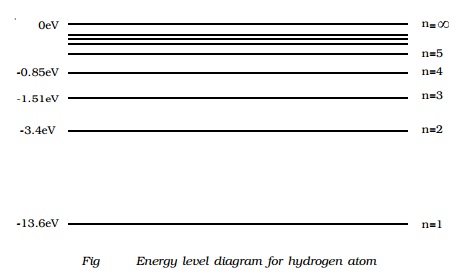
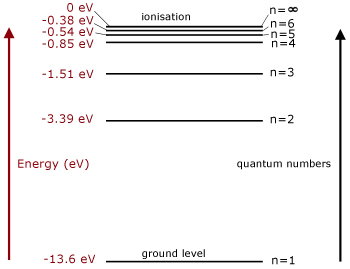
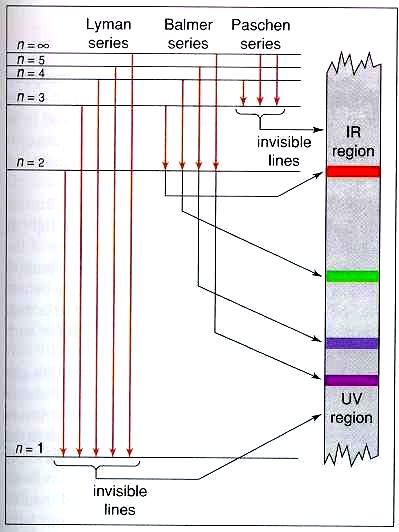
Energy level diagram hydrogen. Energy level diagram . The energy of the electron in the n th orbit of the hydrogen atom is given by, En = -13.6 /n 2 eV. Energy associated with the first orbit of the hydrogen atom is, E 1 = -13.6 /1 2 = -13.6 eV. It is called ground state energy of the hydrogen atom. Energy associated with the second orbit is given by, E 2 = -13.6 /2 2 = -3.4 eV Filters , hydrogen spectrum , HII regions and more ... - posted in Eyepieces: Hi readers , I would like to pass on this info that Ive learnt recently . I was surprised that it was none other than the Balmer series of hydrogen spectrum that contributes to the H-Beta (486nm) , H-Alpha (656nm) . Theres H-gamma , H-epsilon and others but these arent that strong in HII regions . As a class, models of the hydrogen atom are explored using an online java applet in this activity. Real-time spectrometer outputs, visual representations, and energy level diagrams (where appropriate) of the following models are compared and contrasted: Billiard Ball, Plum Pudding, Classical Solar System, Bohr, deBroglie, and Schr ö dinger. The activation energy of the forward reaction is measured from the energy level of ammonia to the top of the 'hump'. This is greater than the activation energy of the backward reaction, which is measured from the energy level of nitrogen gas and hydrogen gas to the top of the 'hump'. Therefore, the two activation energies are different.
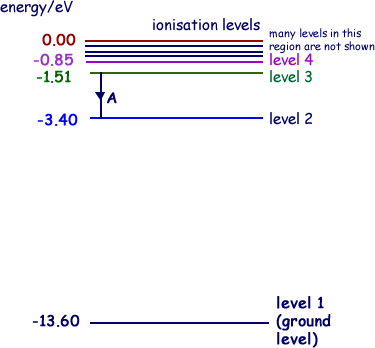
The diagram below shows the energy level diagram of a hydrogen atom. The associated spectrum to the diagram above is shown below. The transition labelled A in the top diagram gives the spectral line labelled B in the spectrum diagram. (a) (i) Show that the frequency of spectral line B is about 4.6 × 10 14 Hz. ΔE = 3.40-1.51 = 1.89 eV. That energy must be exactly the same as the energy gap between the 3-level and the 2-level in the hydrogen atom. The last equation can therefore be re-written as a measure of the energy gap between two electron levels. The greatest possible fall in energy will therefore produce the highest frequency line in the spectrum. The greatest fall will ... Ground state configuration - an atoms electron configuration at the lowest energy level Aufbaus Principle Paulis Exclusion Principle and Hunds Rule must be followed to achieve ground state. Here is a schematic orbital diagram for a hydrogen atom in its ground state. In writing the electron configuration for Iron the first two electrons will go ... A Note: The first half of lithium's electron configuration is written using only "1s 2 " and not "1s 1 1s 2 " because between Hydrogen and Helium, the energy level and orbital do not change. Only the electron occupancy changes, which we denote by changing the superscript from 1 to 2.
Hydrogen binds more strongly to the T d-C 40 isomer than the D 2-C 40 (although it does not, this time, adopt the triple-pentagon tetrahedrally symmetric sites). This lowers the relative energy difference between the isomers until, at C 40 H 5, there is a transition and the T d-C 40 H 5 isomer becomes the most stable. This demonstrates that the ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction 3 January 1995. Unofficial release. Includes most of the existing recent critically evaluated NIST data on atomic energy levels, transition probabilities, and wavelengths. Energy level data are included for over 500 spectra for the elements Na through S, K through Ni, Cu, Ge, Kr, and Mo, La through Lu, and O II. The basic hydrogen energy level structure is in agreement with the Bohr model. Common pictures are those of a shell structure with each main shell associated with a value of the principal quantum number n. This Bohr model picture of the orbits has some usefulness for visualization so long as it is realized that the "orbits" and the "orbit radius" just represent the most probable values of a ...
When the atom is in excited state one or more electrons go to a higher energy state so electron configuration of the excited atom is different. The helium atom contains two protons and two electrons. The electron configuration and the orbital diagram are. Following hydrogen is the noble gas helium which has an atomic number of 2.

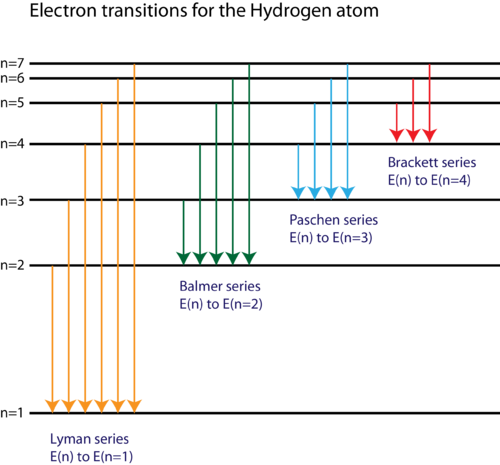
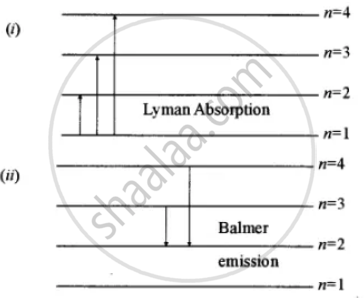
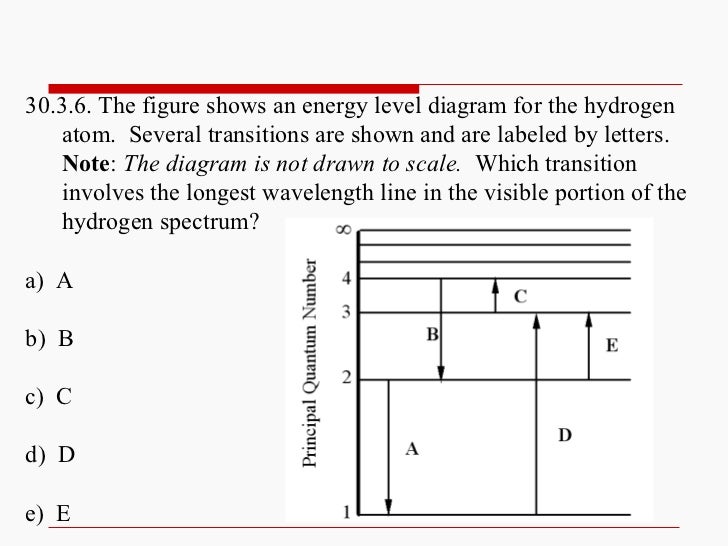
On An Energy Level Diagram Of Hydrogen Show By A Downward Or An Upward Arrow A Transition Which Results In I Emission Line Of Balmer Series Ii Emission Line Of Lyman Series
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing ...
The green arrows indicate the reactions prominent under acidic conditions while the blue arrows indicate the reactions prominent under alkaline conditions. In the schematic diagram of (E), —H indicates that the site on Pt(111) is occupied by hydrogen (H∗), while the rectangle indicates that the site is empty (∗).
Draw Energy Level Diagram For A Hydrogen Atom Showing The First Four Energy Levels Corresponding To N 1 2 3 And 4 Show Transitions Responsible For Physics Theory Shaalaa Com
Basic Hydrogen Calculations of Quantum Mechanics(量子力學有關氫原子的基礎計算) Hydrogen (页面存档备份,存于互联网档案馆) at The Periodic Table of Videos(諾丁漢大學) High temperature hydrogen phase diagram (页面存档备份,存于互联网档案馆)(高溫氫相圖)
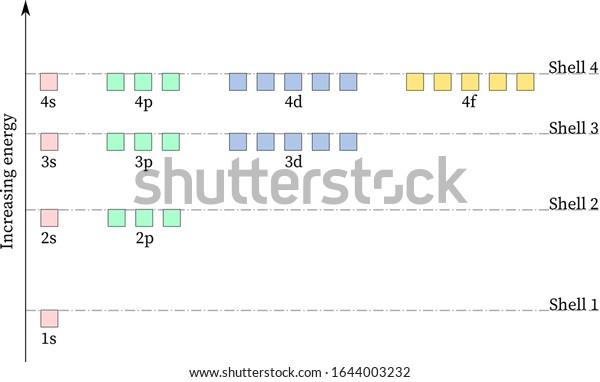
The first quantum number, n, is the electron shell and gives the energy levels of the atomic structure. ... Following the diagram, we can see the subshells are filled in the order 1s, 2s, ...
Hydrogen sulfide is a chemical compound with the formula H 2 S.It is a colorless chalcogen hydride gas with the characteristic foul odor of rotten eggs. It is poisonous, corrosive, and flammable. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestion which is ...
Energy Level Diagram Hydrogen Ppt Powerpoint Presentation Inspiration Icon Cpb Presentation Graphics Presentation Powerpoint Example Slide Templates
When it jumps from a higher energy level to lower energy level it emits energy while it absorbs energy when it jumps from a lower energy level to a higher energy level. The energy absorbed or emitted is equal to the difference between the energies of the two energy levels (E 1, E 2) and is determined by Plank's equation. ΔE = E 2-E 1 = hv ...
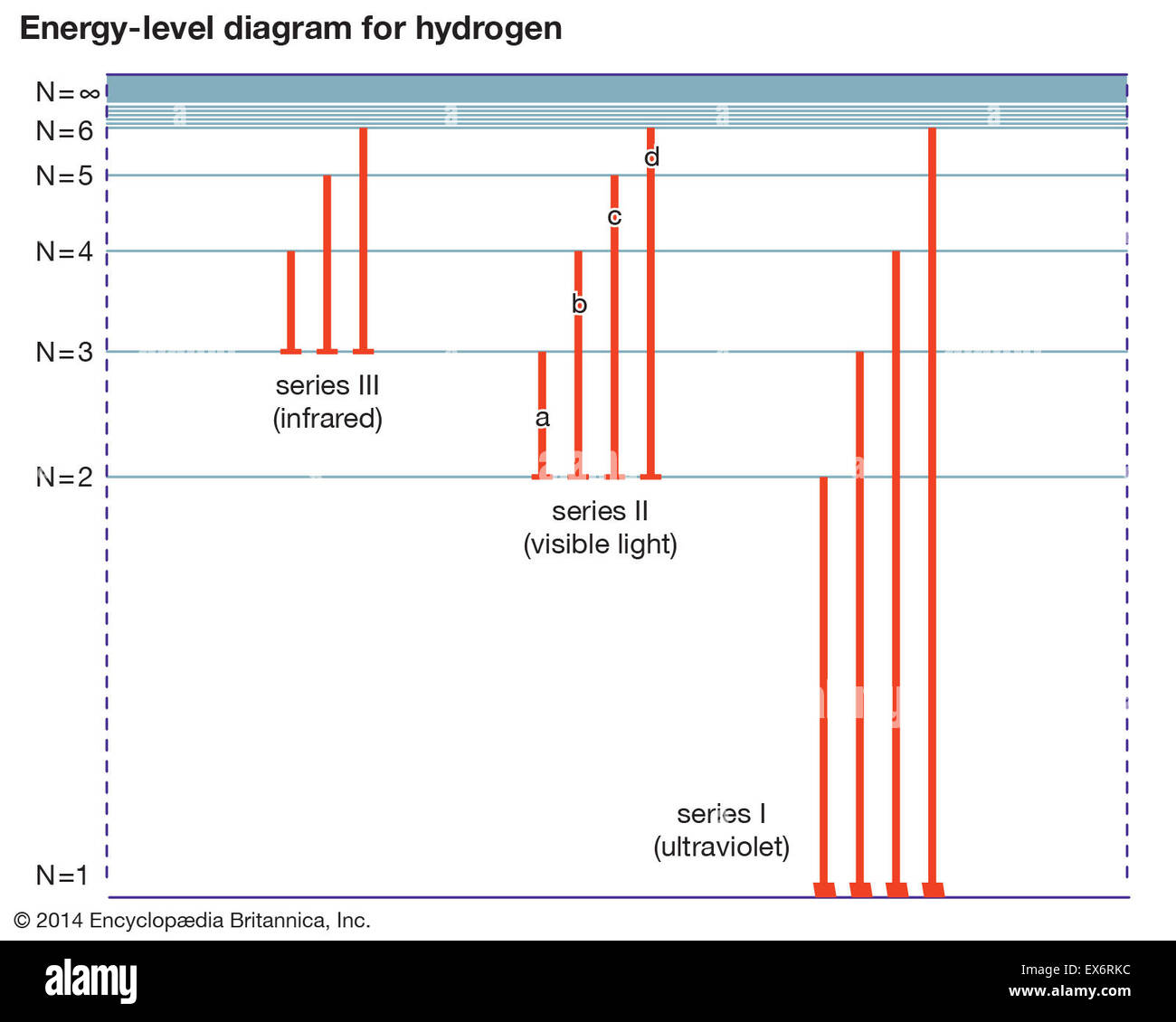
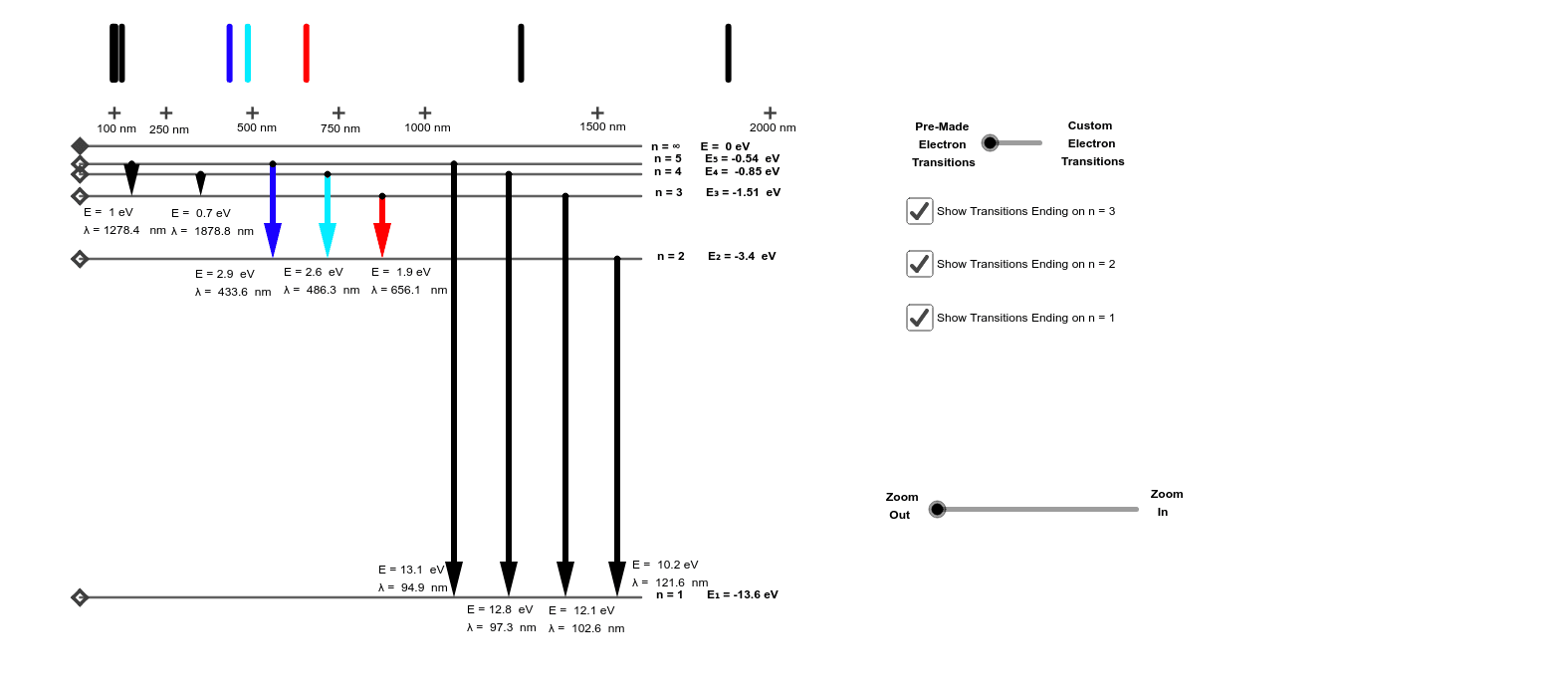
11) Calculate the wavelength of light emitted when each of the following transitions occurs in the hydrogen atom. a) n = 3 -> n=2 b) n = 4 -> n=2 c) n = 2 -> n = 1 12) Using vertical lines indicate the transitions from question 11 on an energy level diagram for the … Continue reading "11) Calculate the wavelength of light emitted when each of the following transitions occurs in the hydrogen ...
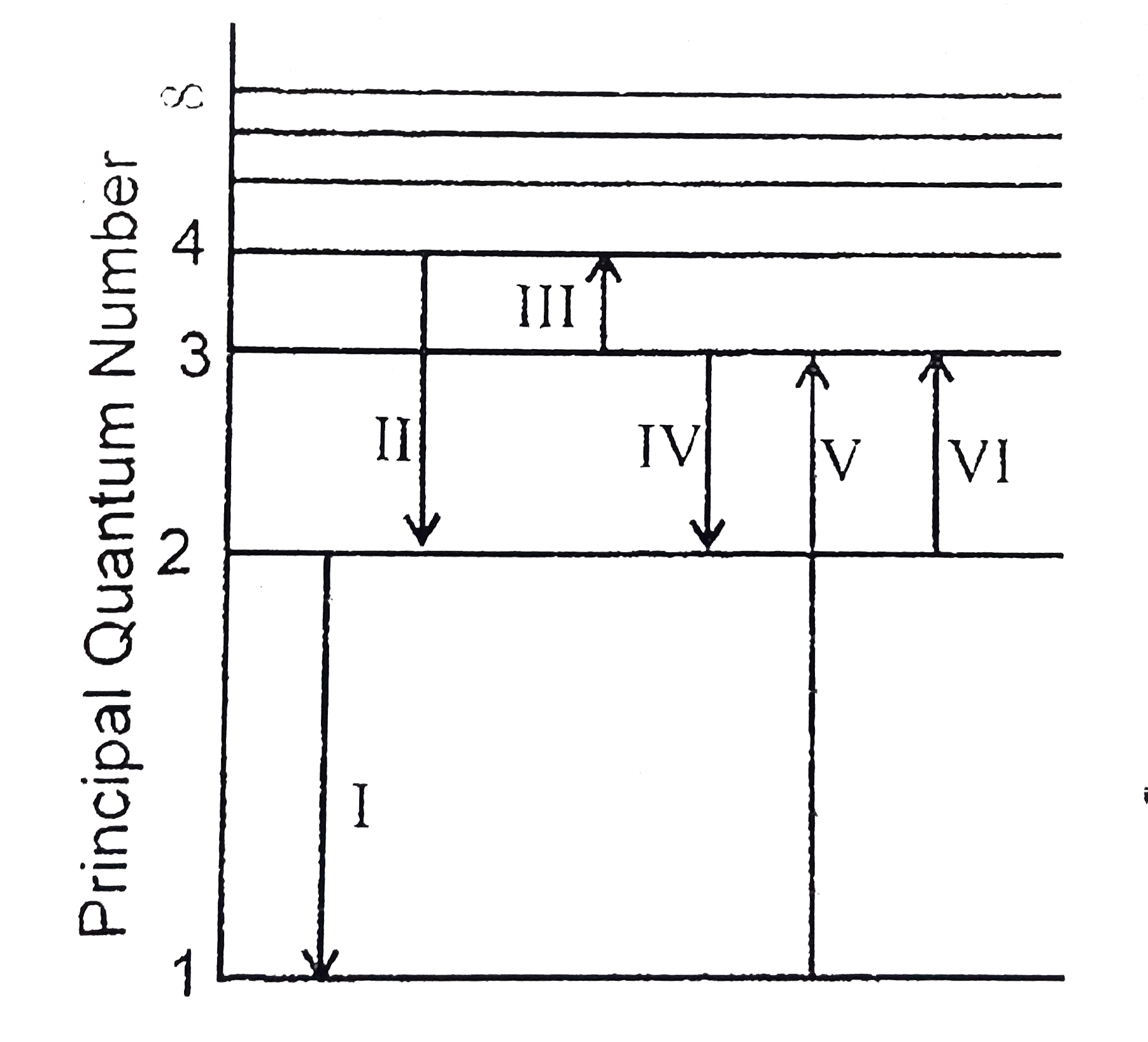
The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transition Are Marked As I Ii Iii The Diagram Is Only Indicative And Not To Scale Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images
reaction. This is because energy is taken in from the surroundings. An upwards arrow shows that energy is taken in Question. Look at the energy level diagrams below.
Apply Hess' Law to the calculation of lattice energy: Sketch energy level diagrams on the basis of given enthalpy changes (Born Haber Cycle) Calculate lattice energy of an ionic crystal from thermochemical data; Given all but one of the enthalpy changes in the Born Haber cycle, calculate the missing value
Oxygen 0 nitrogen n os carbon с phosphorus hydrogen h sulfur electron configuration below is a diagram illustrating electron shells around a chloride ion. Matter has mass and takes up space. Protons have a positive charge, . Drawing atoms worksheet drawing atoms . The 3rd energy level can hold up to 8 electrons.
MO Diagram of HNO3. The sigma bonds between N and O atoms are formed by 2sp2 orbital of nitrogen and a hybrid orbital atom from O atoms. As a result, three sigma bonding and antibonding orbitals are formed. The single sigma bond between hydrogen and oxygen use 1s orbital of hydrogen 2sp3 orbitals of oxygen.
At the same theoretical level, the structural optimization and vibration analysis of all critical points of ThO + H 2 O system were carried out, and then the potential energy surface diagram was given as shown in Figure 3. For detailed information see Fig.S2. Download : Download high-res image (294KB) Download : Download full-size image; Fig. 3.
Hydrogen production is a large and growing industry: with as of 2019 about 70 million tonnes of dedicated production per year, larger than the primary energy supply of Germany.. As of 2019 fertiliser production and oil refining are the main uses. About half [citation needed] is used in the Haber process to produce ammonia (NH 3), which is then used directly or indirectly as fertilizer.
The S 2p 3/2 energy level has a peak at 161.15 eV, and the S 2p 3/2 energy level has a peak at 162.35 eV (Fig. 3d). It is noteworthy that the XPS peaks of Zn, Cd and S of ZCNA-5 move towards lower binding energies compared with ZnCdS, which indicates electron transfer from NiAl LDH to ZnCdS.

Draw A Partial Energy Level Diagram For Hydrogen All Wavelengths Are Ending At The N 2 State And The Energy Of The N 2 State Is 545 Aj Wavelengths Nm 411 26 434 77 487 10 658 42 Study Com
Worked example: Drawing energy level diagrams of the combustion of methane. Answer. Step 1: The chemical equation for the complete combustion of methane is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products.
an energy level diagram. • The intervals between the energy level becomes smaller and smaller towards the top. ... Draw the energy levels in a hydrogen atom. ii. Show the electron transitions which produce the above lines. iii. Give the name of this particular series of lines.

















0 Response to "38 energy level diagram hydrogen"
Post a Comment