38 orbital diagram for magnesium
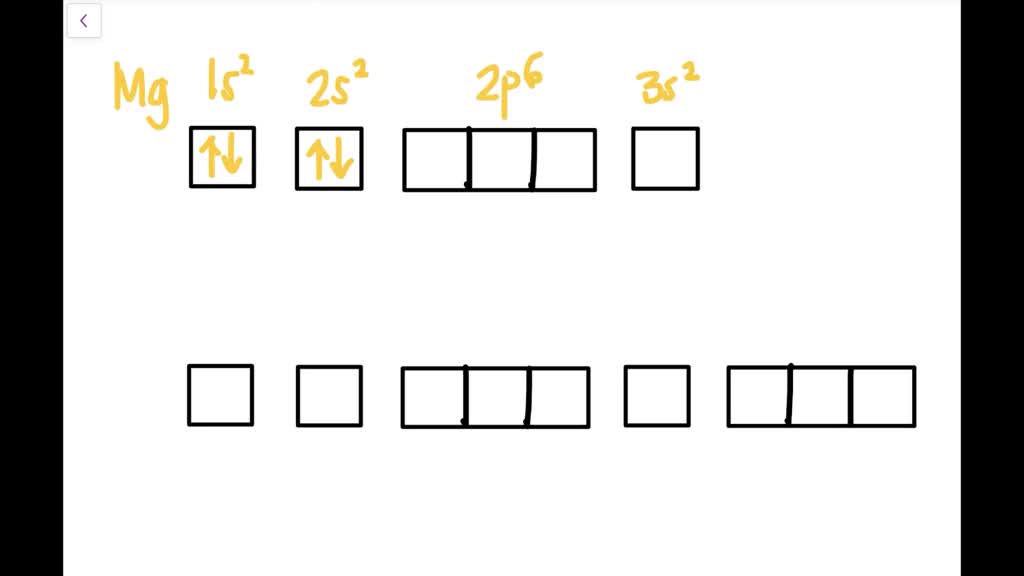
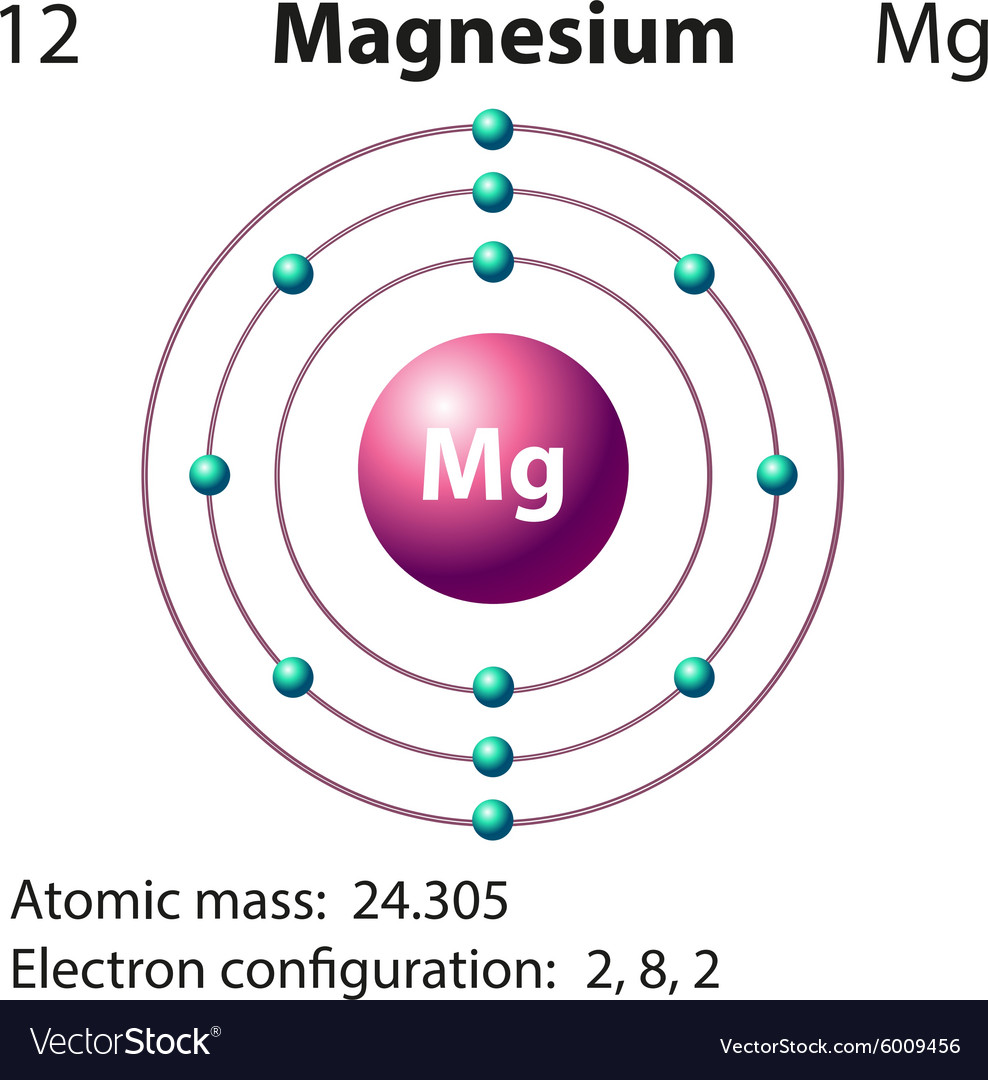
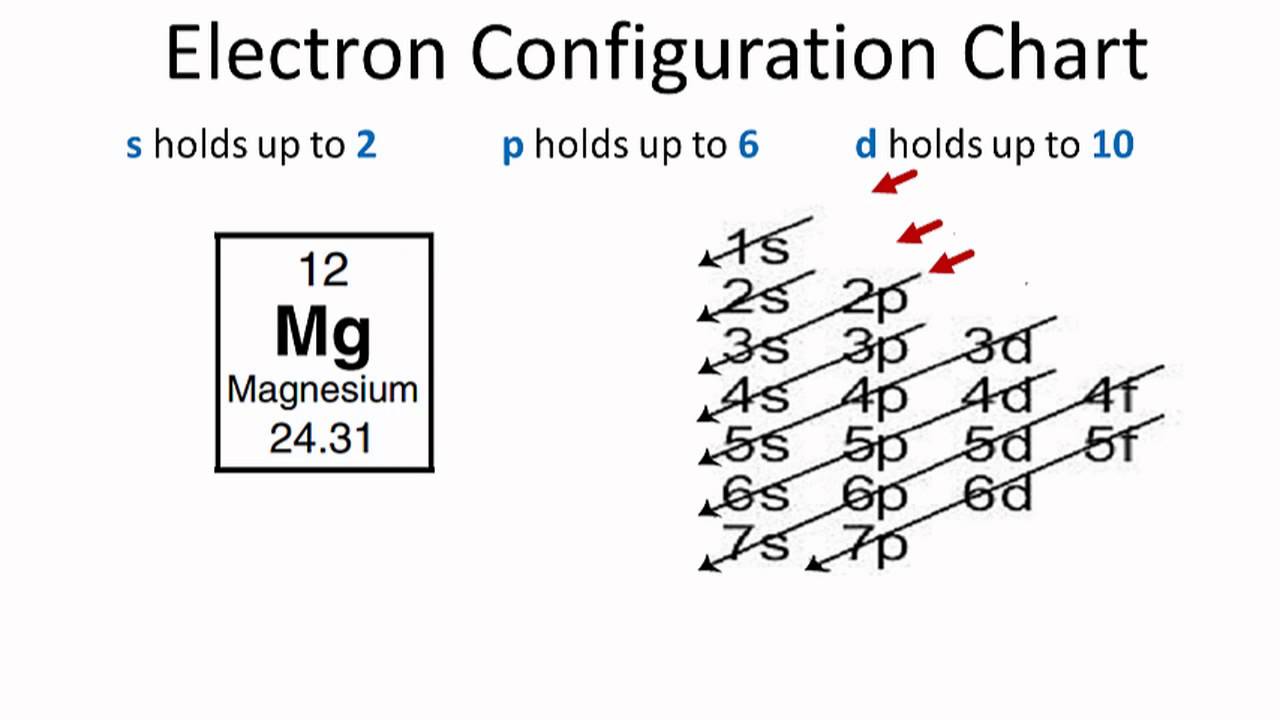
The orbital diagram for MAgnesium is. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down ... The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. soobee72pl and 58 more users found this answer helpful. heart outlined.
Give orbital diagram of the following: magnesium chloride, Medium. Open in App.

Orbital diagram for magnesium
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbital s and hence the boxes, lines or circles, as shown below) 1s. →. 2s. Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
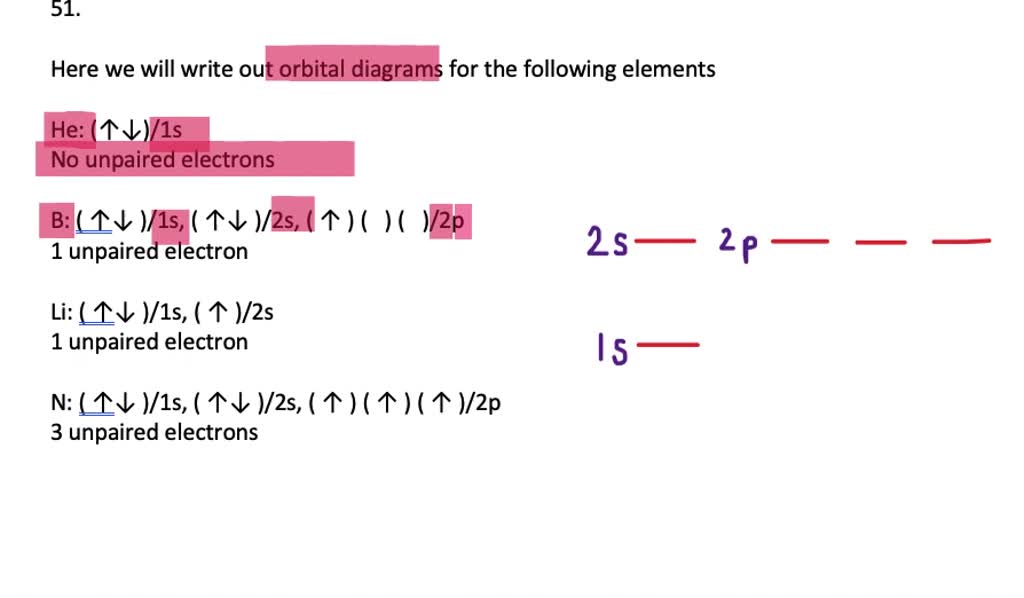
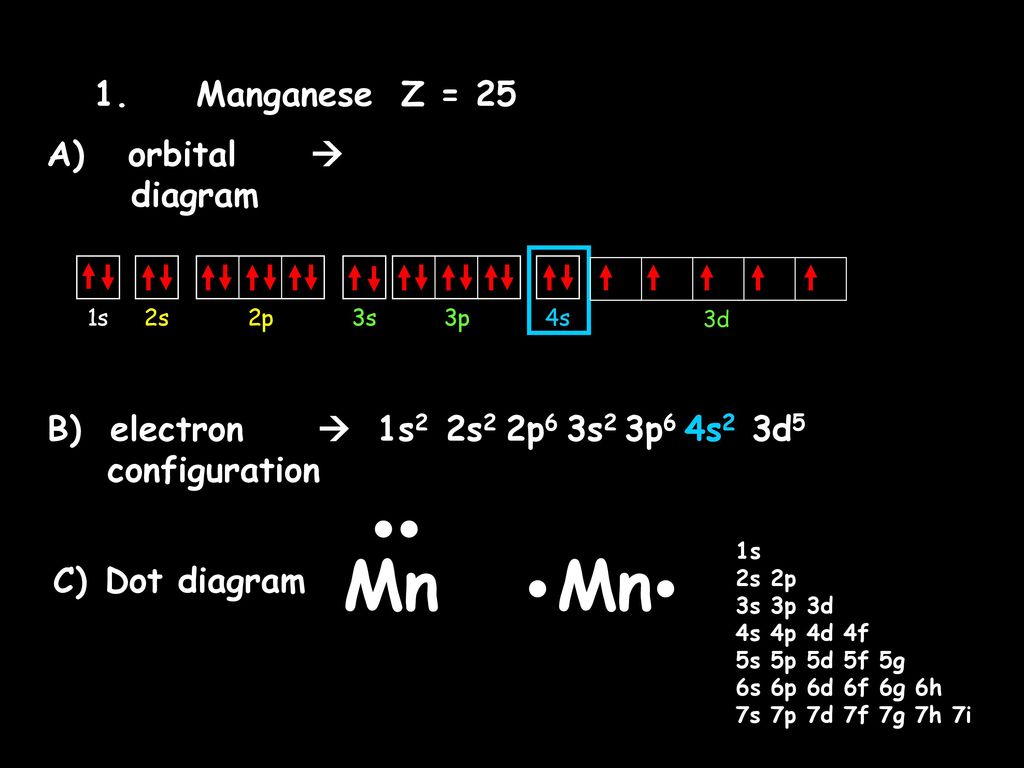
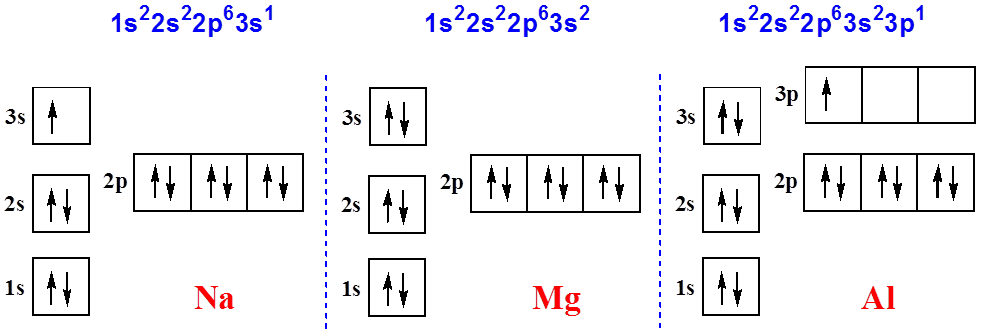
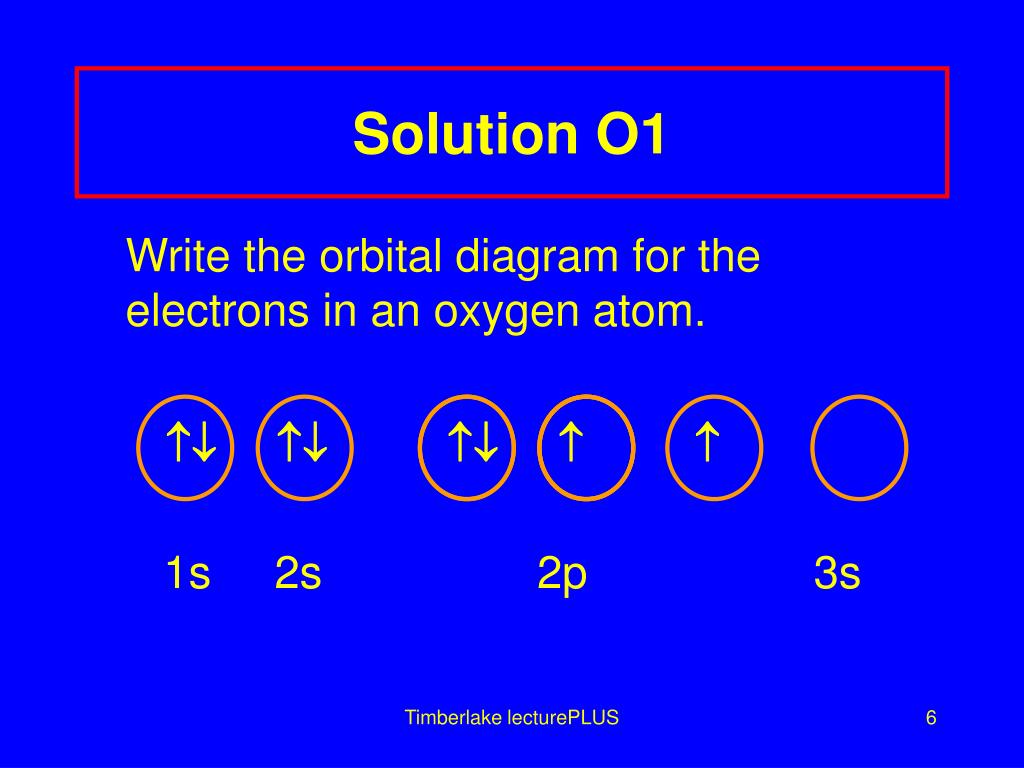
Orbital diagram for magnesium. Write the full orbital diagram for magnesium. Orbital Diagram: The subatomic particle that occupies most of the volume of an atom is known as the electron. The region of space wherein electrons ... Orbital Diagram and Electron Configuration of Magnesium, Continued. 4. Use the diagram to write the electron configuration. Write the number of electrons in each set as a superscript next to the name of the orbital set. 1s22s22p63s2 1s 2s 2p 3s 3p Nov 07, 2021 · Electron Shielding and Effective Nuclear Charge. If an electron is far from the nucleus (i.e., if the distance \(r\) between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure \(\PageIndex{1}\)). Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease ... Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
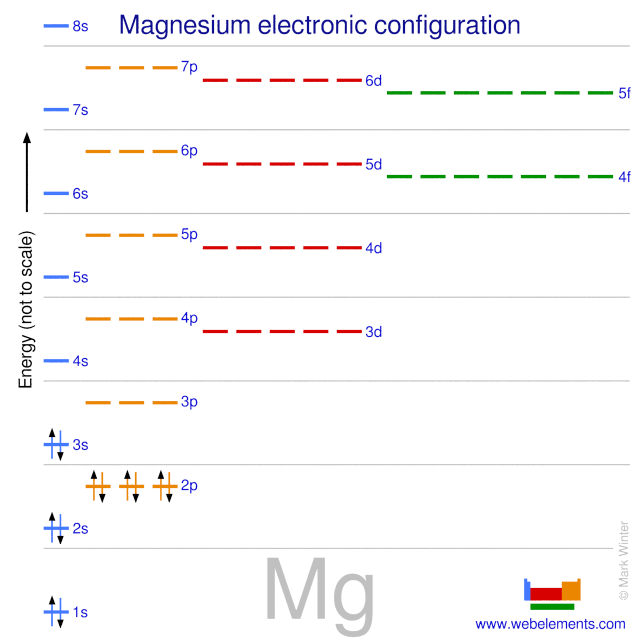
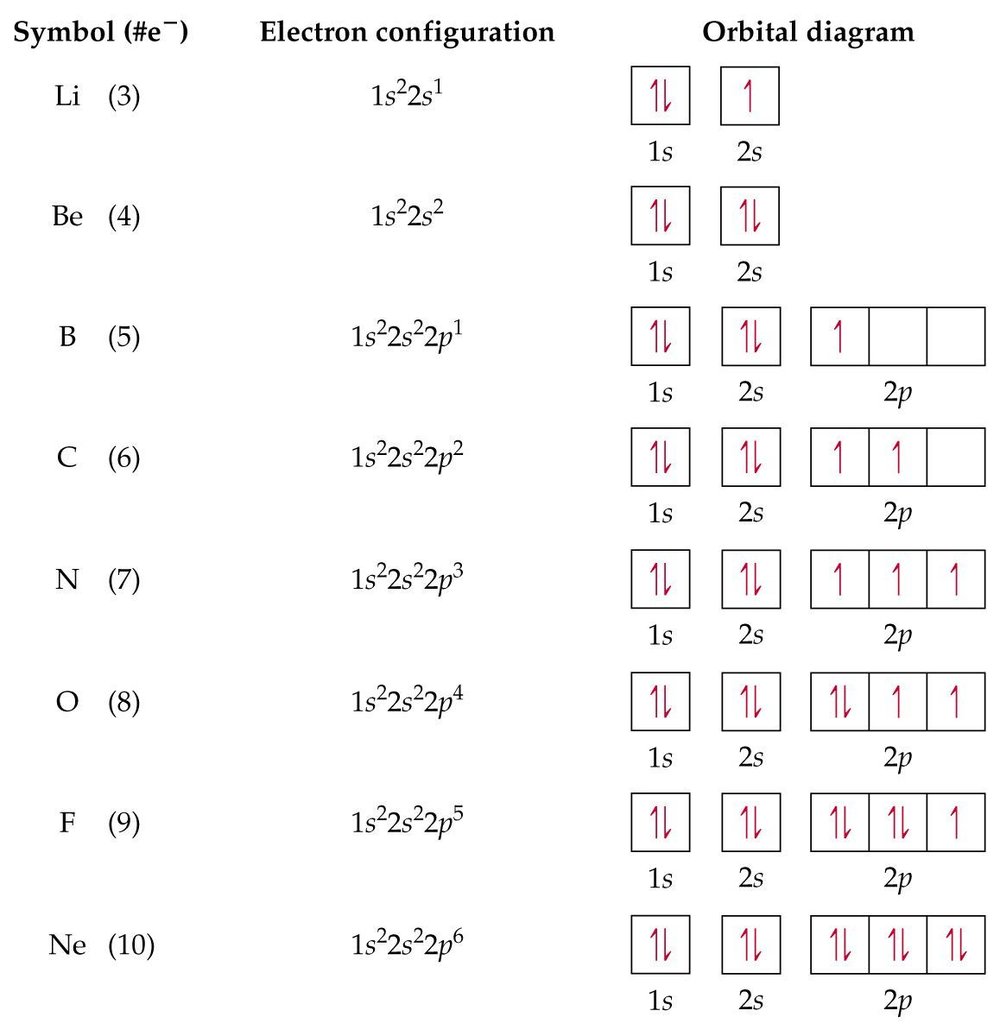
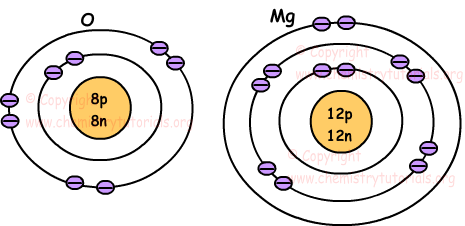
Orbital energy diagrams are provided to guide you learn about the atomic orbital. The following orbital energy diagrams are available in printable quality to show you the mathematical function of electrons in magnesium, boron trifluoride, helium, and chlorine. Explore the diagrams in the following images, simply click to save and print! Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... The magnesium oxide is a non-metal ionic compound that is formed because of electrostatic forces of attraction where no rules of covalent molecules, such as molecular geometry, hybridization, molecular orbital (MO) diagram, and polarity are followed. The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what... a) The atomic number of magnesium is 12, and its electronic configuration is as follows, 1s 2 2s 2 2p 6 3s 2.Therefore, the orbital diagram for magnesium can be drawn as, What is the orbital diagram and electron configuration of magnesium? We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around ... Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled. View Available Hint(s) Reset Help 10 1s 2s 2p 3s 3p 3 G1 G1 G1 G1 G1 G1 n. G G2 G2 G2 G2 G2
Give the Orbital Diagram of the Following: Magnesium Chloride . CISCE ICSE Class 9. Question Papers 10. Textbook Solutions 19257. Important Solutions 6. Question Bank Solutions 14520. Concept Notes & Videos 431. Syllabus. Advertisement Remove all ads. Give the Orbital Diagram of the Following: Magnesium Chloride - Chemistry ...

Electron Configurations Distributedexplains How Electrons Are Distributed Among An Atom S Orbitals Address Each Part Identifies Part Of An Electron S Address Ppt Download
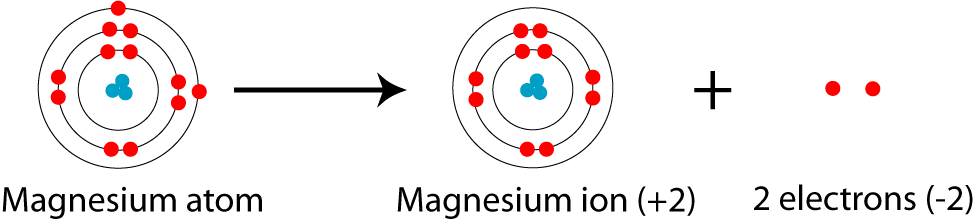
In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We'll also look at why Magnesium forms a 2+ ion and how the electron con...
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbital s and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in.
Solved A Write The Electron Configuration Draw The Orbital Diagram Determine The Distinguishing Electron And Determine The 4 Quantum Numbers For The Distinguishing Electron Of The Element Magnesium Mg Write Electron Configurations As
Solved Write The Electron Configurations For Mathrm Mg And Mathrm Ar Using Both Spdf Notation And Orbital Box Diagrams Describe The Relationship Of The Atom S Electron Configuration To Its Position In The Periodic Table

Write The Electron Configuration For Magnesium A 12 And Potassium A 19 Draw Their Orbital Brainly Ph

Tuliskan Konfigurasi Elektron Dalam Bentuk Diagram Orbital Unsur Unsur Berikut A 12mg B 15p C 24cr Mas Dayat

The Atom What Is It Made Of Protons Positively Charged Mass 1 Amu 1 67 X Grams Located In The Nucleus Gives An Atoms Its Identity Ppt Download




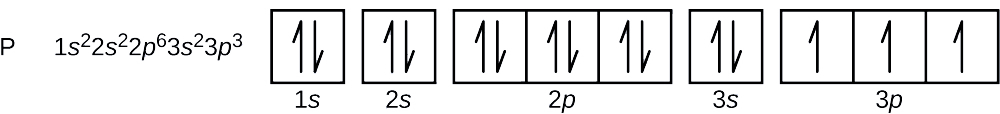











0 Response to "38 orbital diagram for magnesium"
Post a Comment