39 c2 molecular orbital diagram
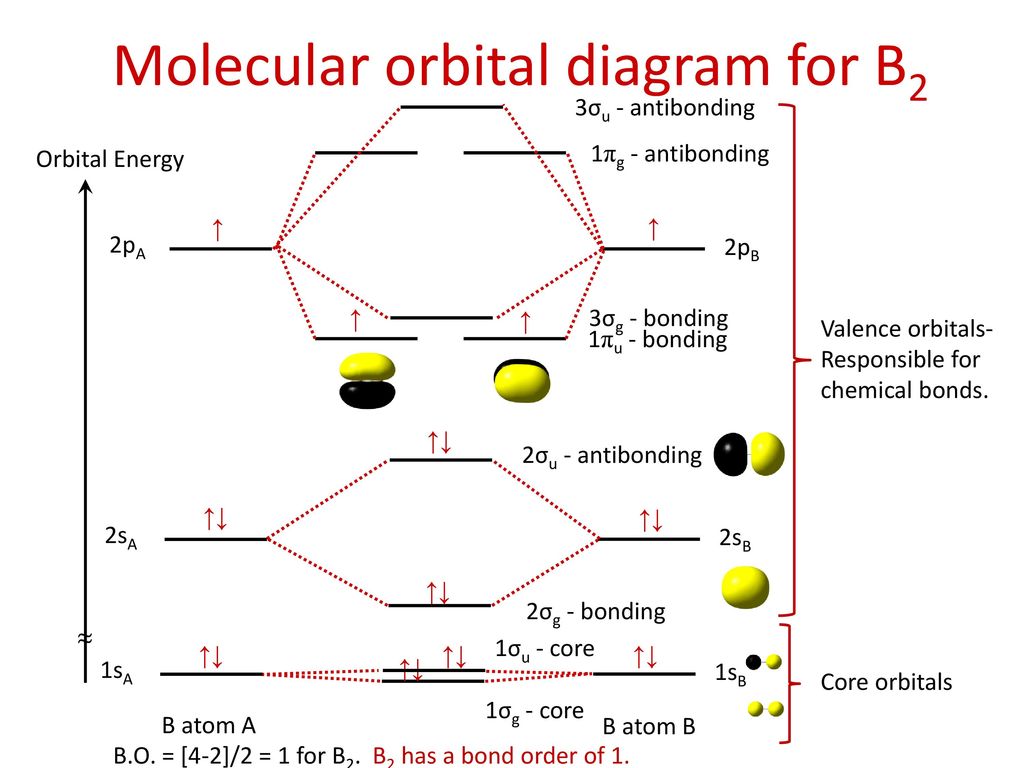
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
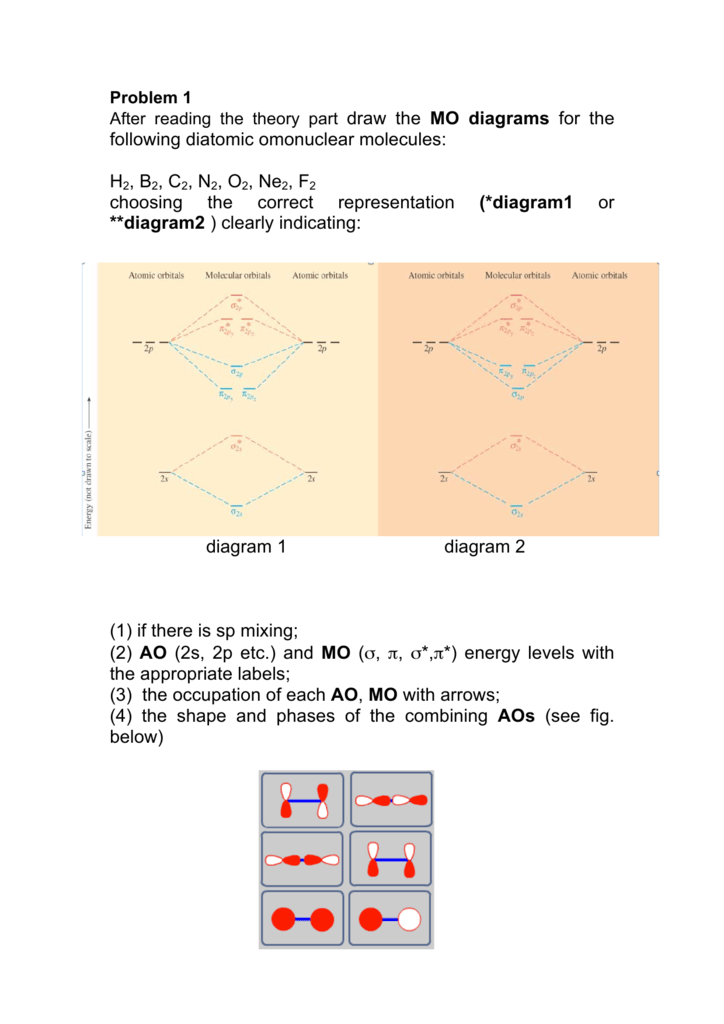
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
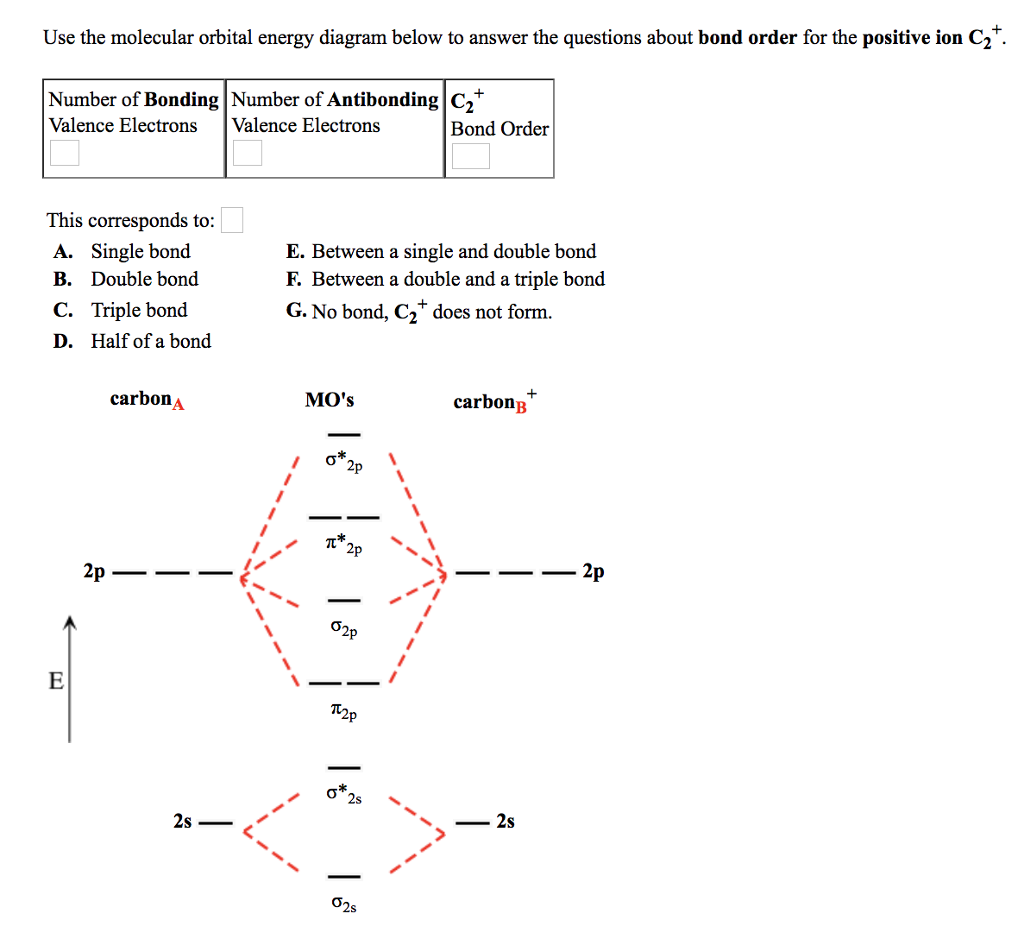
C2 molecular orbital diagram
Molecular orbital diagram for carbon dimer c2. For the ion c22. Give the molecular orbital configuration for the valence electrons in cec22. A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbitals. B calculate the bond order. February 13, 2017 - Answer (1 of 5): By molecular orbital theory, bond order of c2 is 2, while c2 doesn't exist, why? Remember the saying: “Half baked knowledge is dangerous!” Just because some chemical species shows integral value of bond order, doesn’t mean that it should exist. From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
C2 molecular orbital diagram. Molecular orbital diagram for c2. Construct the molecular orbital diagram for he2. Construct the molecular orbital diagram for he2. In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the ... Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics C2 n2 o2 and f2 molecules diego troya. Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for the molecule oxygen o2. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p. At the left of the table the energies are very close to each other and as a result the 2s and 2p orbitals mix with ...
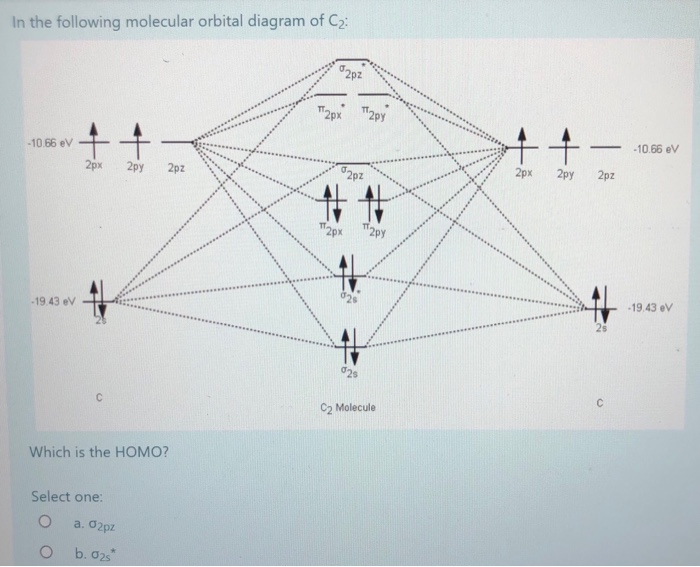
Problem: Use MO diagrams to rank C 2−, C2, and C2+ in order of increasing bond energy. ... Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. May 7, 2018 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
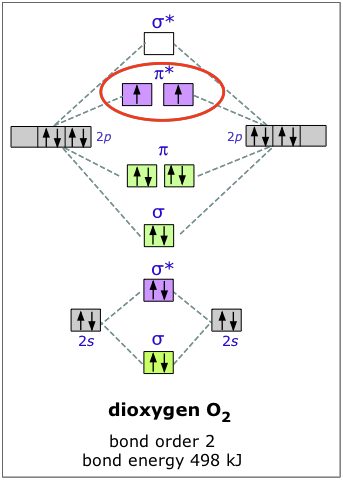
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Mar 26, · This video shows the MO diagrams of the C2, N2, O2 and F2 molecules.A molecular orbital (MO) energy level diagram - Parkway C-2Use the molecular orbital ... C2 2 molecular orbital diagram. Ion predictions with mo diagrams. Interact and form molecular orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals doing this normally just c2 is 128 42. Molecular orbital diagram for carbon dimer c2. Give the molecular orbital configuration for the valence electrons in cec22. Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
This, of course, implies that a ... orbital, LUMO, that follows that the highest energy occupied molecular orbital/highest occupied molecular orbital, HOMO. The diagram below shows the two $ 2p\pi $ orbitals, let's say $ 2p\pi x $ and $ 2p\pi y $ , are the highest energy ...
Molecular orbital (MO) theory explains the construction of molecular orbital diagram on the basis of following main points . 1.Formation of MOs: Atomic orbitals(AOs) linearly combine with each other to form equal number of molecular orbitals (MOs). 2.Energy of MOs: Half of the molecular orbitals (MOs) having energy lower than the atomic orbitals are called…
Molecular orbital diagram for n2 o2 c2 f2 also h2o this problem has been solved. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Orbitals of similar but unequal energies can interact if they have the same symmetry the 2s and 2pzorbitals form mos with the same symmetry σ g and σ u.

Molecular Orbital Theory Chemistry Libretexts In 2021 Chemistry Textbook Geometry Worksheets Electron Configuration
June 20, 2019 - write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order.
FREE Answer to 5. Draw Molecular Orbital Diagrams for C2, C2 and C2. a. Determine the bond order for...
September 9, 2016 - The answer is C2- because of bond orders When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2*[(#e- in bonding orbitals)-(#e- in antibonding orbitals)] Doing this, normally ...
November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
The bond order of C2+ is A 1 B 2 C dfrac32 D dfrac12
Molecular orbital diagram for carbon dimer c2. C2 molecular orbital diagram. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization. Before we can draw a correlation diagram for b2 we must first find the in phase and out of phase overlap combinations for borons atomic orbitals.
Answer to Draw the molecular orbital (MO) electron diagram for the C2+ molecular ion. Be sure your diagram contains all of the ele...
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...

Write Molecular Orbital Configuration Of C2 Predict Magnetic Behaviour And Calculate Its Bond Order H7ch14qq Chemistry Topperlearning Com
Which one of the following statements about C2 molecule is wrong AThe bond order of C2 is 2 BIn vapour phase C2 molecule is diamagnetic CDouble bond in C2 molecule consists of both pi bonds because of
Solutions for Chapter 11Problem 32E: For each of the species C2+, O2−, F2+, and NO+,(a) Write the molecular orbital diagram (as in Example).Example(b)Determine the bond order, and state whether you expect the species to be stable or unstable.(c) Determine if the species is diamagnetic or ...
FIG. 1. The 1σg (left panel) and 1σu (right panel) molecular orbitals of C2 The next two molecular orbitals (2σg and 2σu) corresponds to the symmetric and, re-spectively, antisymmetric linear combinations of the 2s orbitals on each C atom. Note that the 2σu orbital also contains a substantial contribution from the 2pz (l = 0) orbitals (the 2p
Answer (1 of 5): By molecular orbital theory, bond order of c2 is 2, while c2 doesn't exist, why? Remember the saying: "Half baked knowledge is dangerous!" Just because some chemical species shows integral value of bond order, doesn't mean that it should exist. If it doesn't, it means we need ...

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.
The formal bond order calculated with these orbitals and occupation numbers is 2 (resulting from 6 electrons in bonding orbitals and 2 in an antibonding orbital). Figure 2. Schematic picture of the molecular orbital diagram obtained from MO theory. In VB, the bond picture arises from considering that the C atom bears a sp hybridization.
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
C2 molecular orbital diagram. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Get 11 help now from expert chemistry tutors. Fill from the bottom up with 8 electrons total. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms.

The Photoelectron Spectrum Of C2 Has Not Yet Been Measured Sketch A Predicted Spectrum Based On The Molecular Orbital Homeworklib
Molecular orbital diagrams of diatomic molecules. Then we rank them in order of increasing energy. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. For o2f2 and n2 the sigma bond comes first then the pi bo. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p.
From Wikipedia, the free encyclopedia. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) ... So in C2; We can see both the Carbons are Doubly bonded,thus the Bond Order in C2 ...
October 15, 2019 - Click here👆to get an answer to your question ✍️ According to molecular orbital theory, C2 molecule has :

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Diatomic carbon (systematically named dicarbon and 1λ 2,2λ 2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written [C 2] or C 2).It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and ...
From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
February 13, 2017 - Answer (1 of 5): By molecular orbital theory, bond order of c2 is 2, while c2 doesn't exist, why? Remember the saying: “Half baked knowledge is dangerous!” Just because some chemical species shows integral value of bond order, doesn’t mean that it should exist.
Molecular orbital diagram for carbon dimer c2. For the ion c22. Give the molecular orbital configuration for the valence electrons in cec22. A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbitals. B calculate the bond order.

















0 Response to "39 c2 molecular orbital diagram"
Post a Comment