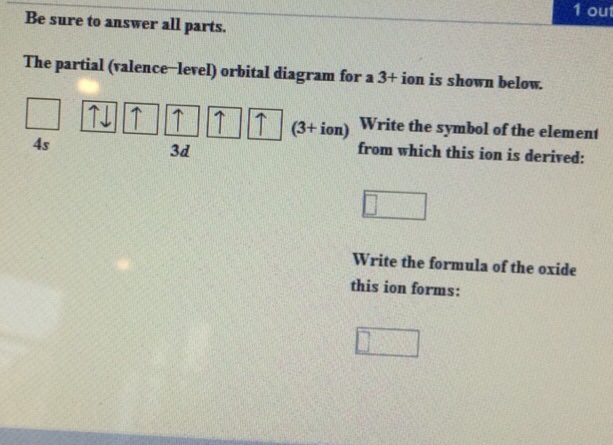
40 the partial (valence-level) orbital diagram for a 3+ ion is shown below.
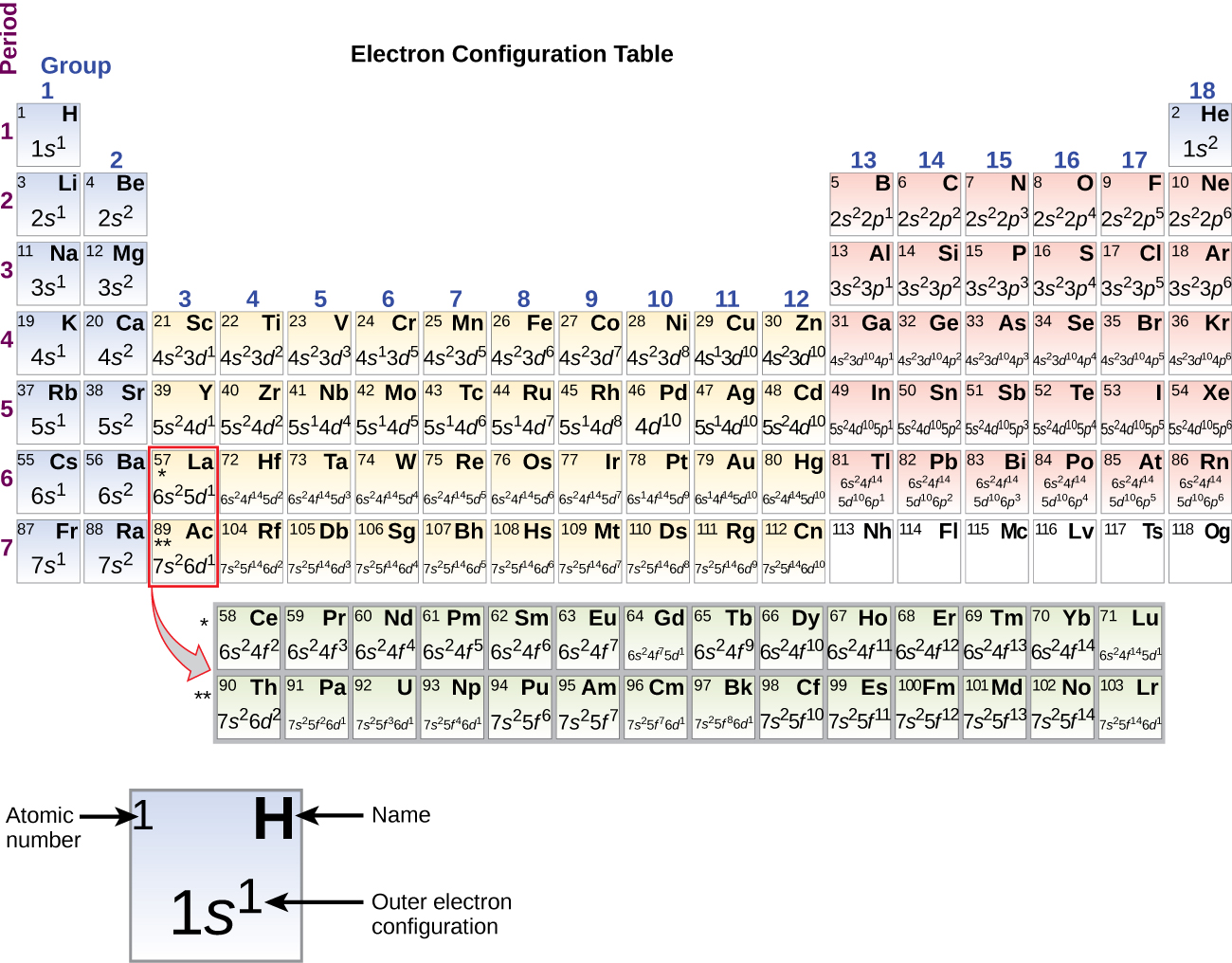
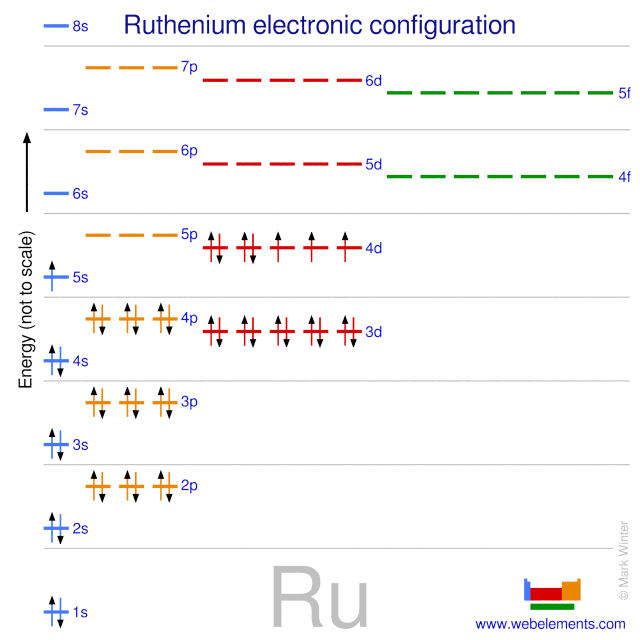
Electron configurations of transition metal elements Hydrogen Z 1. Lithium Z 3. Ce 3 Xe4f 1. Iron which forms either the Fe 2 or Fe 3 ions loses electrons as shown below. The Electron Configuration of Transition-Metal Ions. CeriumIII leadII Ti 2 Am 3 Pd 2 For the examples that are transition metals determine to which series they belong. The sublevel 2p has 3 orbitals (2p x, 2p y, and 2p z) and each of these orbitals has its own line (or box). Let's begin this section with the orbital box (or the orbital representation diagram) for a neutral atom. Draw the electronic configuration for potassium using the electron configuration diagram below. Remember that potassium is element ...
BeCl2 Lewis Structure. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any molecule. These electrons will be both bonding as well as non-bonding electrons. The electronic configuration of beryllium is [He] 2s2and chlorine is [Ne] 3s23p5. The number of electrons on the valence shell of Be and Cl is 2 ...
The partial (valence-level) orbital diagram for a 3+ ion is shown below.
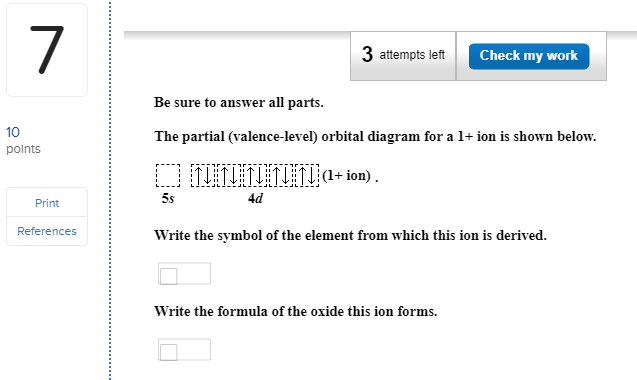
Figure 6.8. 1: One electron in. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l ( s, p, d, or f ), with the number of electrons in the subshell indicated by a superscript. The location of the electrons within the various orbitals is often expressed by orbital diagrams and electron configuration symbols. Choose from Radium Electron Configuration stock illustrations from iStock. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 7s 2 Back to key information about the element. 10 polnts The partial (valence-level) orbital diagram for a 1+ ion is shown below. + ion 4d Print References Write the symbol of the element from which this ion ...
The partial (valence-level) orbital diagram for a 3+ ion is shown below.. Electron orbital diagram s and written configurations tell you which orbital s are filled and which are partially filled for any atom. The number of valence electrons impacts on the ir chemical properties, and the specific ordering and properties of the orbital s are important in physics, so many students have to get to grips with the basics. Construct the orbital Diagram for as. The partial (valence-level) orbital diagram for a 3+ ion is shown below. tutt 11 (3+ ion) 4s 3d Write the symbol of the element from which this ion is ... Transcribed image text: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is ... Transcribed image text: The partial (valence-level) orbital diagram for a 2+ ion is shown below. Write the symbol of the element from which this ion is ...
Frontier molecular orbitals diagram of the two orientation isomers of C 70 fullerene anion systems. The pristine anion is calculated at the B3LYP/6-31G (d, p) level including GD3. The others are for the B3LYP/6-31G (d, p) level including GD3 plus the point charges model. This is in line with Madelung's rule, as the 4s-orbital has n + l = 4 (n = 4, l = 0) while the 3d-orbital has n + l = 5 (n = 3, l = 2). After calcium, most neutral atoms in the first series of transition metals (scandium through zinc) have configurations with two 4s electrons, but there are two exceptions. 26 Jan 2021 — Ti tanium Electron Configura ti on: Ti tanium is a chemical element that has a chemical symbol Ti.Its atomic number is 22. Ti tanium (Ti) has an atomic mass of 22.Find out about its chemical ... Electron Configura ti on, [Ar] 3d2 4s2. 1s2 2s2 2p6 3s2 3p6 3d2 4s2. Orbital Diagram.The compound must be cyclic; Each element within the ring must have a p-orbital that is ... The hybridization of carbon monoxide is sp as its geometrical structure is linear. The below mention diagram is the valence shell electronic configuration of both the carbon and oxygen atom. The half-filled sp(z) hybrid orbital of the carbon atom head-on overlaps with the half-filled sp(z) hybrid orbital of the oxygen atom.
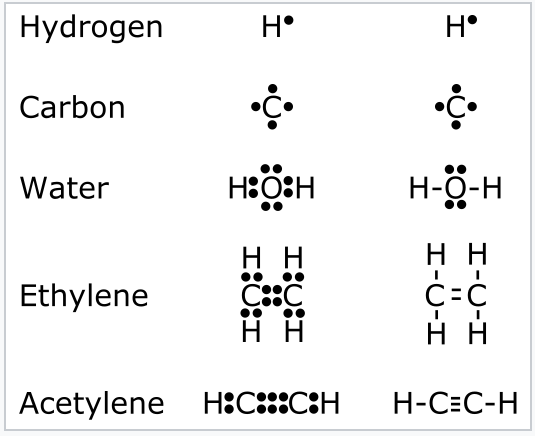
The representation of valence electrons are explained below: Electron Dot Diagrams. As valence electrons are significant to an atom's reactivity, it is important to represent them by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN). An Introduction to Electron Configurations. Electron Configuration An electron configuration is a description of electron arrangement within an atom, which indicates both population and location of electrons among the various atomic orbitals.. General Rules for Electron Configurations 1. Electrons occupy orbitals of the lowest energy available. 26 Mar 2021 · 1 answerQ175587. The partial (valence-level) orbital diagram for a 3+ ion is shown below. __ 4s (no arrows) __ __ __ __ __ 3d (two arrows in the ...
BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.
The bond order can be interpreted from MO diagram s using the following for mula: `" Bond Order" = 1/2 [(" Bond ing "e^-)-("Anti bond ing " e^-)]` One half the difference between the number of electrons present in the bond ing and the anti-bond ing orbitals is bond order Bond order (B.O) =1/2(Nb−Na) Bond order of H2− To tal number of ...
This approach is used only when the group orbitals are not obvious by inspection. Science. Chemistry. Chemistry questions and answers. Assignment Score: 36.9% Resources Hint Check Answer < Question 7 of 16 >; A partial MO diagram is shown for CN. Place the 2p atomic orbital for carbon in the correct location Which orbital in the diagram is the HOMO?
The partial (valence-level) orbital diagram for a 2+ ion is shown below. quifditu1 (2+ion) 5s 4d Write the symbol of the element from which this ion is derived.

Solved Partial Valence Level Electron Configurations For Four Different Ions Are Shown Below Identify The Elements From Which The Ions Are Derived And Write The Formula Of The Oxide Each Ion Forms
How many valence electrons does molybdenum have? (give only 1 answer) Go to this site and look for the electrons available in the outside shells. Click on element #42 and scroll down the menu on the left side until you come to electron configuration. Chemistry. 1) What is the charge on the ion that Selenium forms in an ionic compound?
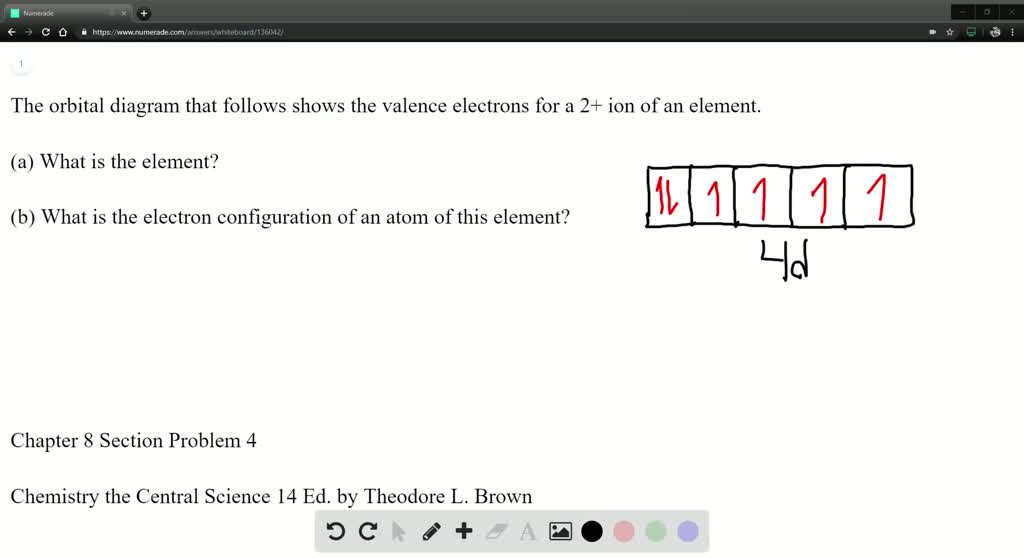
Solved The Orbital Diagram That Follows Shows The Valence Electrons For A 2 Ion Of An Element A What Is The Element B What Is The Electron Configuration Of An Atom Of This
36 construct the orbital diagram of the f- ion. Written By Christine J. Bell Tuesday, November 16, 2021 Add Comment. Edit. Construct the orbital diagram of each atom or ion. In writing the electron configurat ion for fluorine the first two electrons will go in the 1s orbital. The 24 electrons of a.
Transcribed image text: The partial (valence-level) orbital diagram for a 3-ion is shown below. What is the symbol of the element from which this ion is ...
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbital s and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
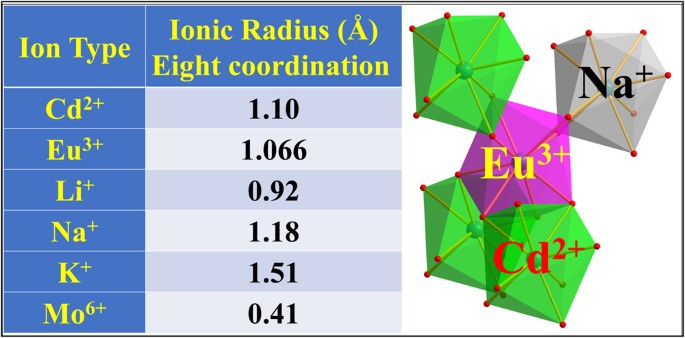
Break The Interacting Bridge Between Eu3 Ions In The 3d Network Structure Of Cdmoo4 Eu3 Bright Red Emission Phosphor Scientific Reports
Transcribed image text: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is ...
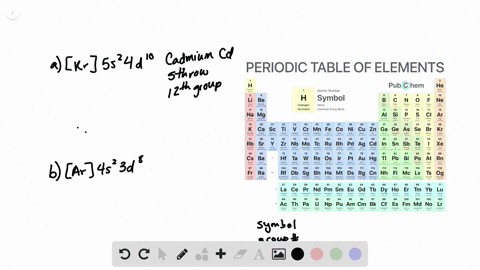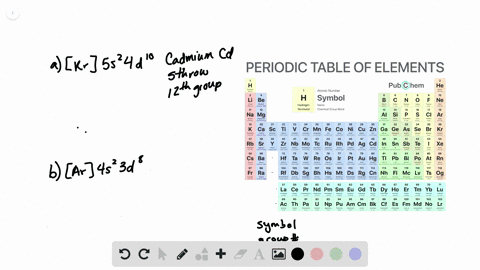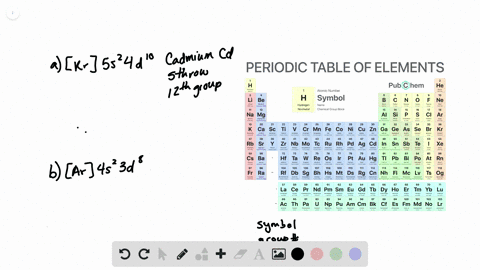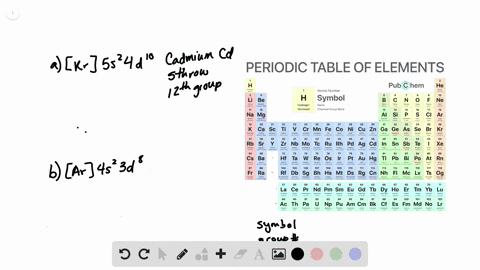
Solved Electron Configuration And Chemical Periodicity Chemistry The Molecular Nature Of Matter And Change 2016 Numerade
Now further, I have explained all the Periodic trends with images and I have also given you a simple trick to remember each trends in Periodic table. Let's dive straight into it. But before that if you want to jump to your interested topic, then click on the below topics. #1 Periodic trends: Valency.
Transcribed image text: The partial (valence-level) orbital diagram for a 4+ ion is shown below. What is the symbol of the element from which this ion is ...
The electron configurat ion of a neutral magnesium atom is: 1s2 2s2 2p6 3s2 or in shorthand [Ne] 3s2. Magnesium has 2 valence (outer-shell) electrons and will lose both to fulfill the octet rule ... The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block ...

Solved Attempts Ieft Check My Work Be Sure To Answer All Parts The Partial Valence Level Orbital Diagram For A 2 Ion Is Shown Below Ihihwi Hile Ion Write The Symbol Of The Element From Which
Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration. February 18, 2021 by Sneha Leave a Comment. Vanadium Electron Configuration: When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned be for e in our article. Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a ...
Carbon He2s 2 2p 2. An orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with up and down. This table is available to download as a PDF to use as a study sheet. The electron configuration 1s22s22p1 is the ground state electron configuration of. 1s 2 2s 2 2p 1.

Wadsley Roth Crystallographic Shear Structure Niobium Based Oxides Promising Anode Materials For High Safety Lithium Ion Batteries Yang 2021 Advanced Science Wiley Online Library
Alex Molecular Orbital Electron Configuration Written By Kitts Romen1994 Monday, November 22, 2021 Add Comment Edit. Chapter 8. Advanced Theories of Covalent Bonding. 8.4 Molecular Orbital Theory . Learning Objectives. By the end of this section, you will be able to:
Molecular Orbital Theory An approach, known as Molecular Orbital Theory, was established primarily by Hund and Mulliken in \(1932\) to explain the features of molecules such as their relative bond strengths, paramagnetic and diamagnetic properties, etc.
1 is the principal quantum number or energy level shell s is the sub-level or sub shell Capacity of s sub shell is 2 electron 2 shows the number of electrons in the s sub shell. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. Spell out the element name that matches the electron configuration. The principal quantum number is denoted n and can.
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.

Solved Partial Valence Level Electron Configurations For Four Different Ions Are Shown Below Identify The Elements From Which The Ions Are Derived And Write The Formula Of The Oxide Each Ion Forms
The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Ozone consists of three oxygen atoms. Oxygen belongs to group VI of the periodic table with an atomic no of 8. It thus has 6 valence electrons. Thus, the total number of valence electrons in ozone= 3*6 = 18
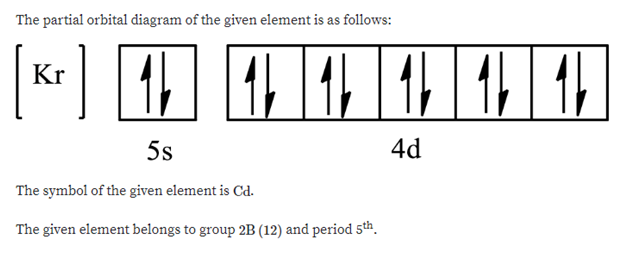
The partial (valence level) orbital diagram for a 2+ ion is shown below... Then it shows a box with no arrows for the 5s orbital (aka no electrons) then it shows the 4d orbital with five boxes the first 3 boxes have an up and down arrow in each box (indicating the electrons spin (spin up spin down)) and the last 2 boxes only have spin up arrows.
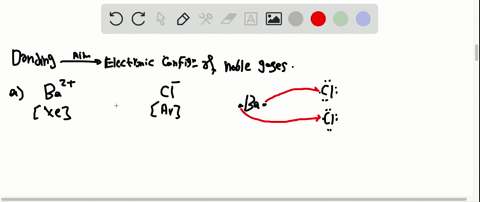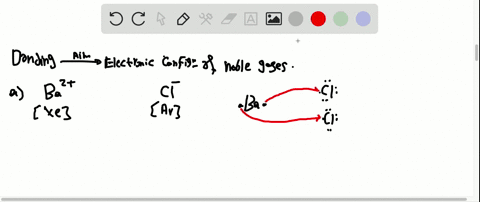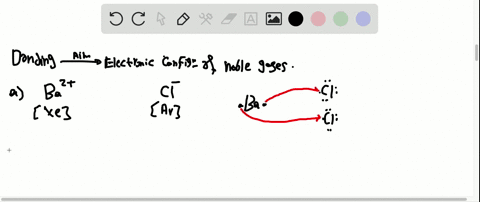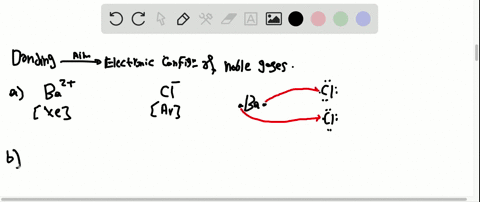
Solved Partial Valence Level Electron Configurations For Four Different Ions Are Shown Below Identify The Elements From Which The Ions Are Derived And Write The Formula Of The Oxide Each Ion Forms
10 polnts The partial (valence-level) orbital diagram for a 1+ ion is shown below. + ion 4d Print References Write the symbol of the element from which this ion ...
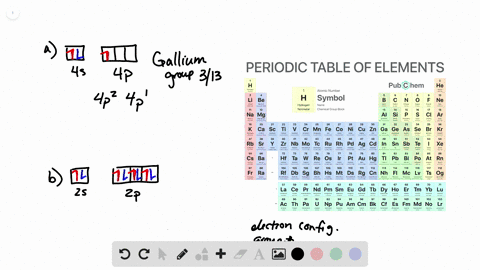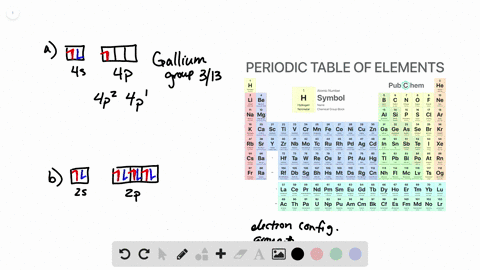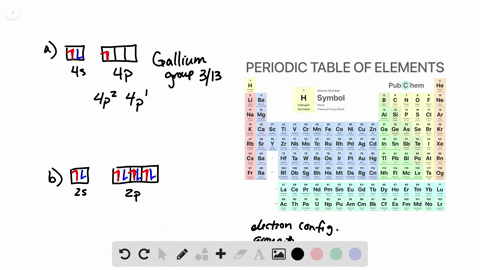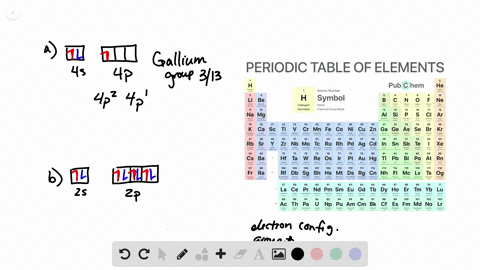
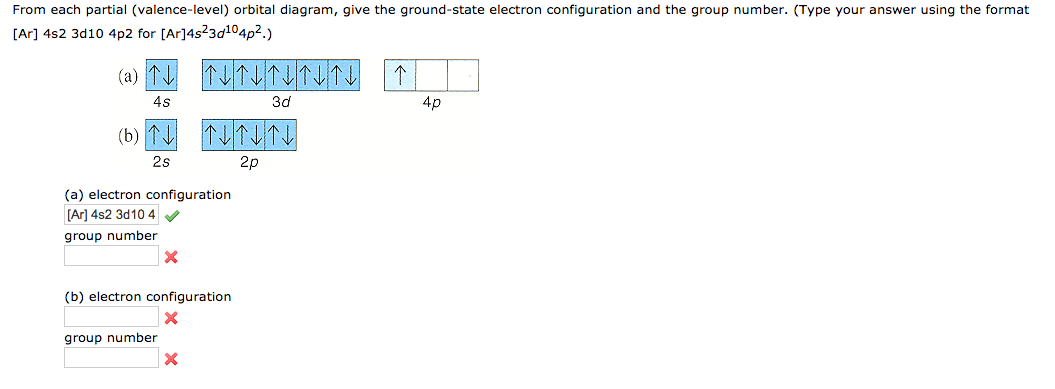
Solved From Each Partial Valence Level Orbital Diagram Write The Condensed Electron Configuration And Group Number A B
The location of the electrons within the various orbitals is often expressed by orbital diagrams and electron configuration symbols. Choose from Radium Electron Configuration stock illustrations from iStock. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 7s 2 Back to key information about the element.
Figure 6.8. 1: One electron in. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l ( s, p, d, or f ), with the number of electrons in the subshell indicated by a superscript.
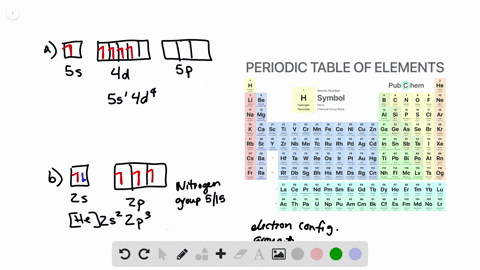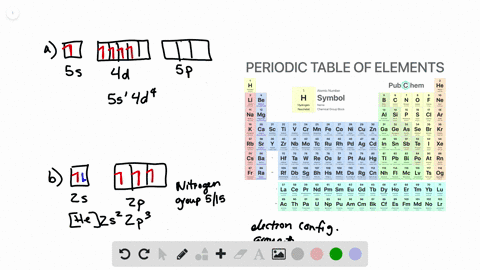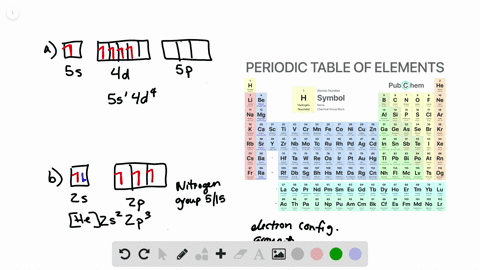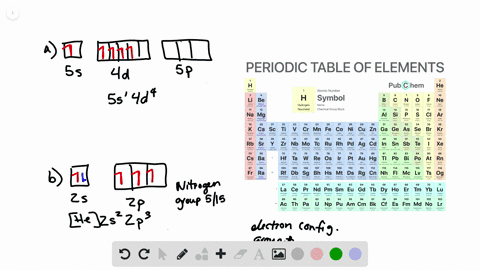
Solved The Orbital Diagram That Follows Shows The Valence Electrons For A 2 Ion Of An Element A What Is The Element B What Is The Electron Configuration Of An Atom Of This

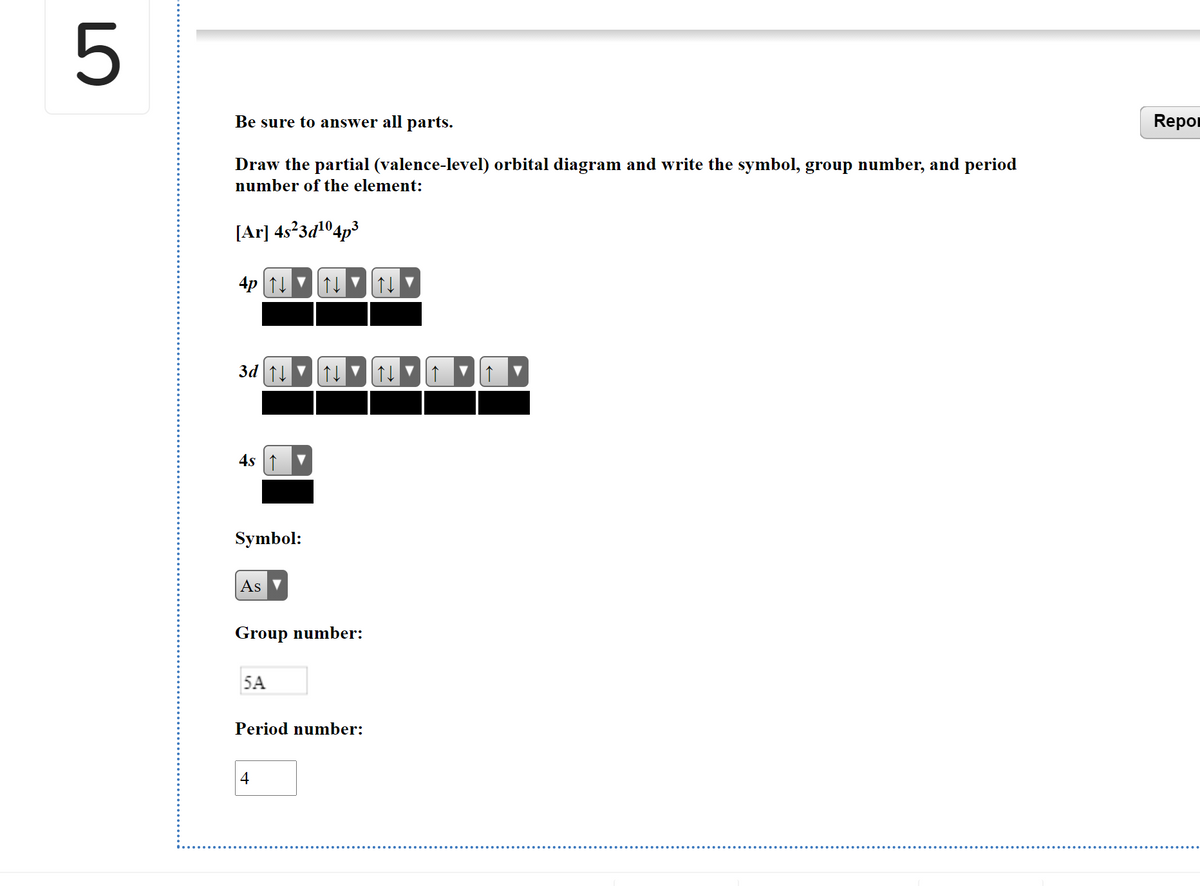
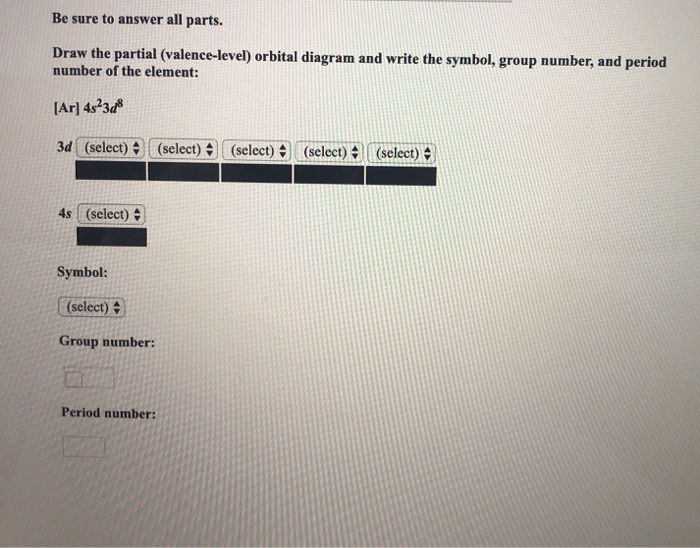
Draw The Partial Valence Level Orbital Diagram And Write The Symbol Group Number And Period Number Of The Element Ar 4s 2 3d 10 4p 3 Image Src Orbital9195593143458043682 Jpg Alt Orbital C Study Com

Progress And Perspectives Of Electrochemical Co2 Reduction On Copper In Aqueous Electrolyte Chemical Reviews

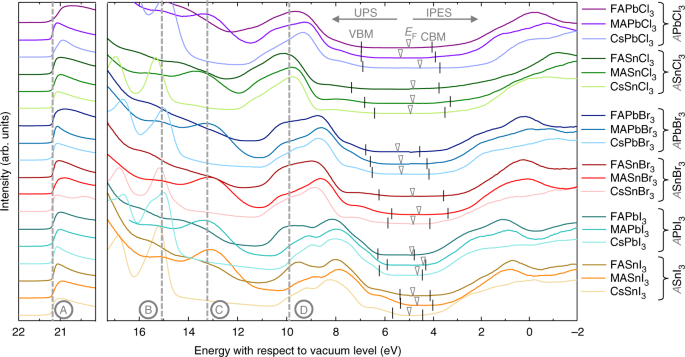



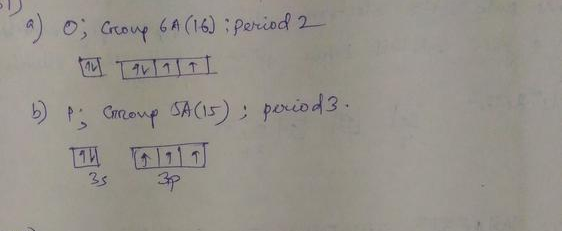



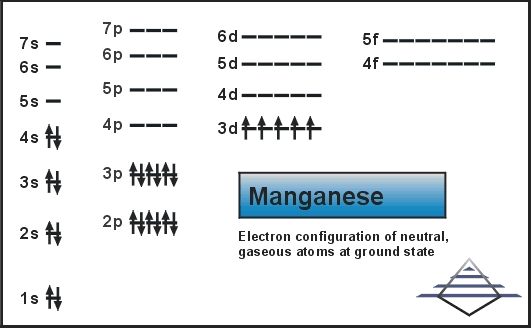





0 Response to "40 the partial (valence-level) orbital diagram for a 3+ ion is shown below."
Post a Comment