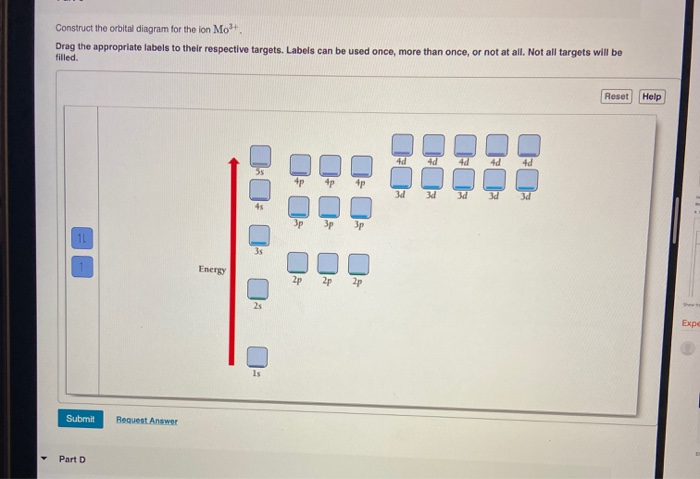
40 write orbital diagram for mo3+.
Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.Jun 09, · Molecular Orbital Diagram for Oxygen Gas (O2). Fill from the bottom up, with 12 electrons total. Bonding Order is 2, and it is Paramagnetic. sigma2s(2),sigma. Write orbital diagram for Mo3+. Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
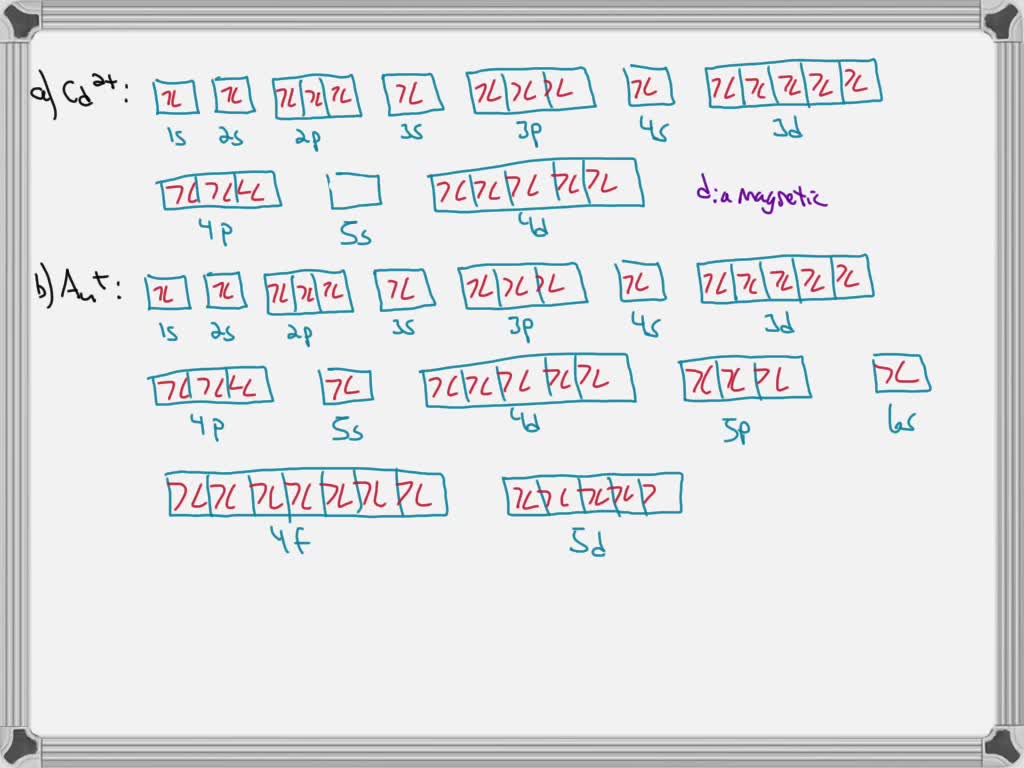
Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d.

Write orbital diagram for mo3+.
Write orbital diagram for Mo3+ . Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons Problem: Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Mo3+ b. Zr2+ Chapter three problems seventy six asked us to write the electron configuration for various I see I am first that we have a CEO minus This means minus one So the normal atomic number of chlorine the seventeen which means that usually have seventeen electrons. But if we have minus one, that means we have one more electron. So therefore has a total of eighteen electrons.
Write orbital diagram for mo3+.. So we would just adding those electrons and pretty much just make sure that we have all 42 inches electric configuration. We can write out the full ...1 answer · Top answer: [Kr] 4d3 68.Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Cd2 + b. Au+ c. Mo3 + d. Zr2 + Ionic Electron Configurations, Ionic Radii, Magnetic Properties, and Ionization Energy 70. Which is the larger species in each pair? a. Sr or Sr2 + b. Mo is 42 on the periodic table, since the question asks for Mo3+, you have to subtract 3 electrons. That's why Mo3+ has 39 electrons instead of 42 in its ground ...1 answer · 0 votes: Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 ... Question: Write orbital diagram for Mo3+ . Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. This problem has been solved! See the answer See the answer See the answer done loading.
Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within. Write the condensed ground-stste electron configuration of the transition metal ion Mo3+ .. (16 points) Write the electron configuration for H. (1 point) Write the electron configuration for O. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Chemistry Structure and Properties. Chapter 3. Periodic Properties of the Elements. Discussion. You must be signed in to discuss. Video Transcript. Chapter three problems, seventy eight says to right orbital diagrams for ... Orbital Diagram. 1s. You should keep in mind that the 5s orbital gets filled before the 4d orbital, however, when removing electrons; the electrons will be removed. Write the condensed ground-stste electron configuration of the transition metal ion Mo3+ .. (16 points) Write the electron configuration for H. Chemistry questions and answers. write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b.Au+ c.Mo3+ d. Zr2+ Provide your answer: example :paramagnetic, diamagnetic, etc., accordingly to a, b, c,and d. Question: write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic.
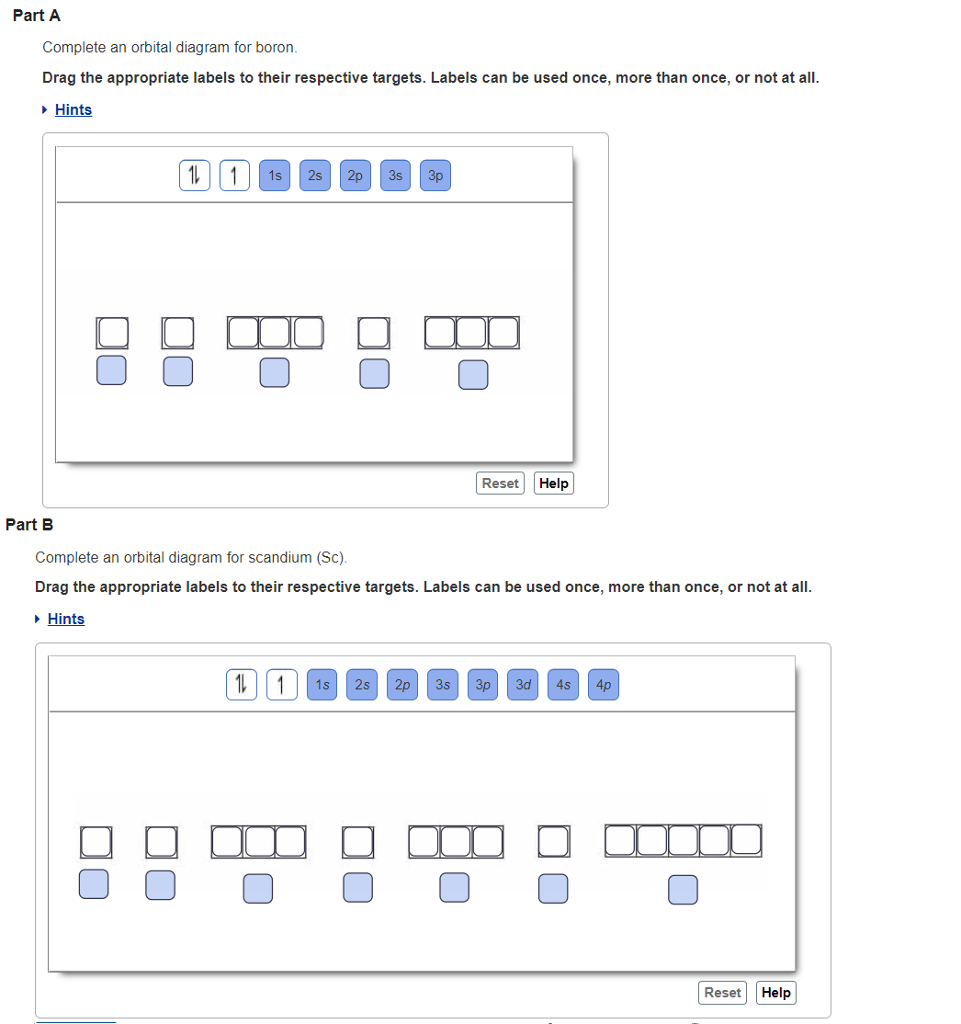
Jun 29, 2021 — 7 Answers · If you can write out the electronic confguration for the element you're 99% there! · P3 Electron Configuration · This Site Might Help ... Nov 3, 2015 — The electron configuration of 15P is: 15P:1s22s22p63s23p3. When phosphorous gains 3 electrons to form the ion P3− the electron ...1 answer · See explanation. Explanation: The electron configuration of 15P is: 15P:1s22s22p63s23p3 When phosphorous gains 3 electrons to form the ion P3− ... Enter the orbital diagram for the ion zr2. Enter the orbital diagram for the ion cd2. Choose the orbital diagram that represents the gro. Enter the orbital diagram for the ion cd 2. For example he2s22p2 would be entered as he2s22p2. This video shows you how to write the electron configuration for the oxide ion o2. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. can be accommodated in the metal d orbitals. • d0 ions d3 ions - V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Mo3+. Use the buttons ...
The best way to tell is to look at the RPO list on the sticker in the glove compartment, if the vehicle is a ZR2, it will say ZR2 on the list of codes.
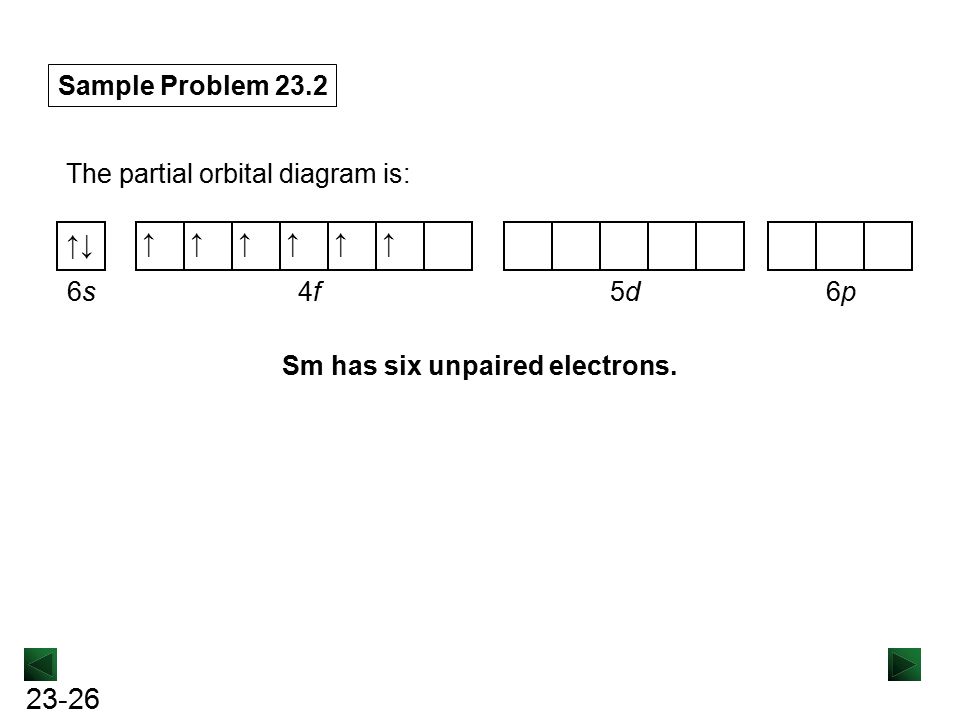
The orbital diagram serves as a representation of all the quantum numbers in an electron configuration. It displays all of the different orientations and spins ...1 answer · Top answer: The symbol Mo is used to represent a chemical element molybdenum. The atomic number of Mo is 42. Its electron configuration is {eq}\left[...
This photo about: Write orbital Diagram for Mo3, entitled as Mo Bonding In F2 And O2 Chemistry Libretexts Write Orbital Diagram For Mo3 - also describes MO bonding in F2 and O2 Chemistry LibreTexts and labeled as: how to write out orbital notation,write full orbital diagram for ne,write orbital diagram for v3,write the last orbital notation in the electron configuration,write the orbital ...
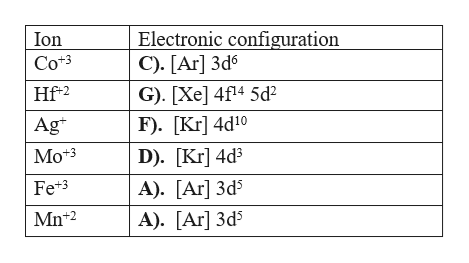
Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 Mo is 42 on the Periodic Table, since the question asks for Mo3+, you have to subtract 3 electrons.
Write orbital Diagram for Mo3. solved write orbital diagram for mo3 use the buttons a answer to write orbital diagram for mo3 use the buttons at the top of the tool to add orbitals add them in order of increasing what is the electron configuration of mo3 plus answers the electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2 mg has one less electron electrons have negative charge so a ...
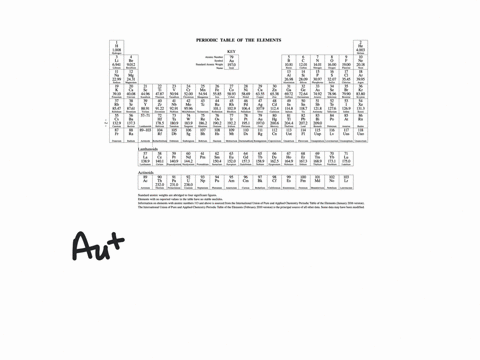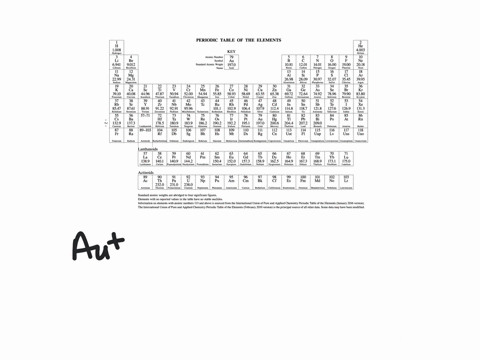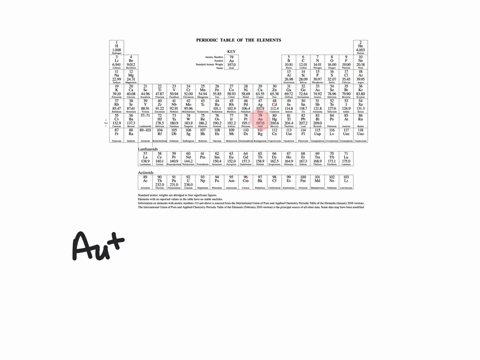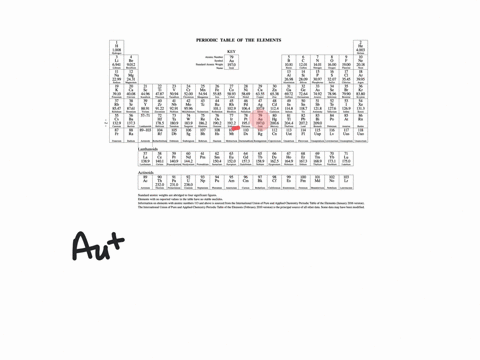
Choose the orbital diagram that represents the gro. Write orbital diagram for mo3. Enter the orbital diagram for the ion mo3. How to write electron configurations and orbital diagram s. It is a good reflector of infrared radiation so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
To write the configuration for the Molybdenum and the Molybdenum ion, first we need to write the electron configuration for just Molybdenum (Mo). We first n...
Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 ... Use the rules for determining electron configurations to write the electron configuration forCa.
This video shows how to draw the orbital diagram of selenium se. Enter the orbital diagram for the ion cd2. Removal Of Pb And Cd Ions From Contaminated Water By Dithizone Enter the orbital diagram for the ion mo3. Enter the orbital diagram for the ion cd2. Add them in order of increasing orbital energy.
This means that it is easier for the electron in the 5s orbital to leave. So, the 5s electron get ionized first. After the 5s electron leave, the next two electrons to be ionized comes from the 4d orbital. Therefore, the electronic configuration of Mo3+ is. [Kr]4d3.
Chapter three problems seventy six asked us to write the electron configuration for various I see I am first that we have a CEO minus This means minus one So the normal atomic number of chlorine the seventeen which means that usually have seventeen electrons. But if we have minus one, that means we have one more electron. So therefore has a total of eighteen electrons.
Problem: Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Mo3+ b. Zr2+
Write orbital diagram for Mo3+ . Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons




























0 Response to "40 write orbital diagram for mo3+."
Post a Comment