34 cs molecular orbital diagram
(b) Develop the MO energy level diagram for 𝐿𝐿𝐿𝐿𝐿𝐿. Clearly indicate which atomic orbitals mix to make which molecular orbitals. Write the orbital occupancy for 𝐿𝐿𝐿𝐿𝐿𝐿, and estimate the bond order. (c) We would expect 𝐿𝐿𝐿𝐿𝐿𝐿 to have substantial ionic character. Does your MO work agree with
Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in L a T e X by means of the package MOdiagram.For information about the more traditional molecular structure diagrams see our documentation about chemistry formulae.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Cs molecular orbital diagram
If we talked about the ground-state Cesium Electron Configuration (Cs), than it is written as the following; 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 1. Now many of the users must be wondering what exactly it is, therefore for them, they have two choices: either they learn the direct electronic configuration or they learn ...
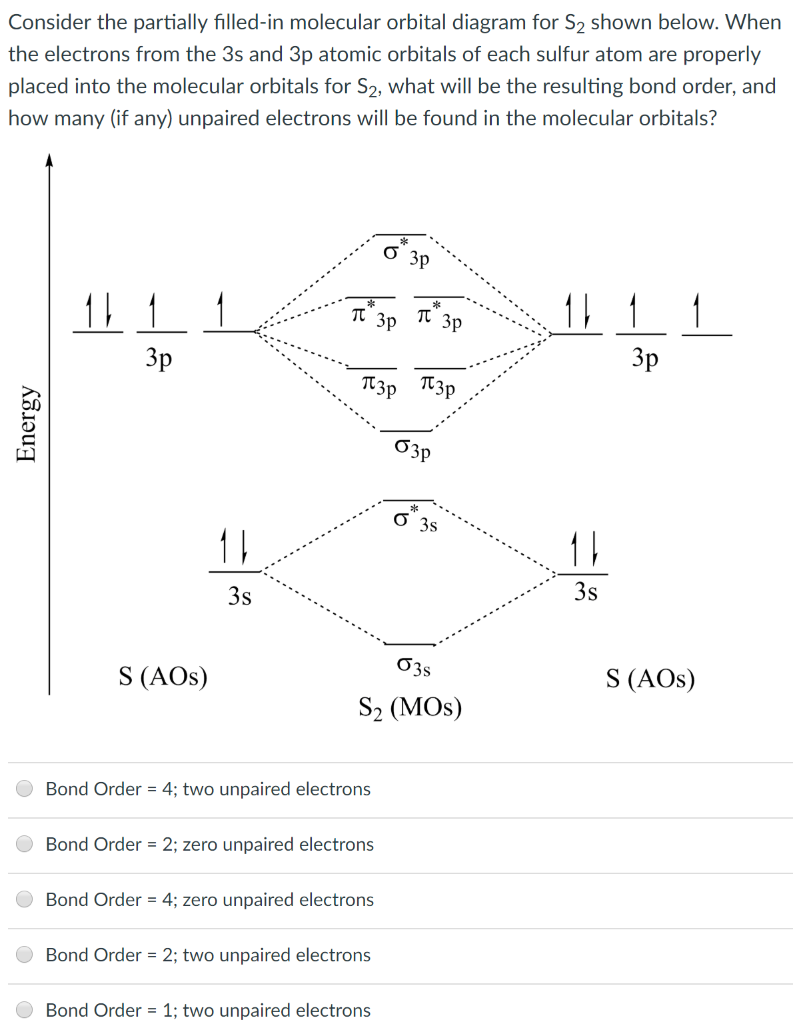
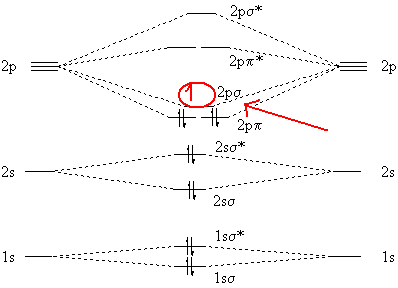
Best Answer. This is the best answer based on feedback and ratings. 100% (34 ratings) Transcribed image text: Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s Click within the blue boxes to add electrons. Previous question Next question.
ORBITALS - are specific regions of space where electrons may exist - The SHAPE of an orbital is defined by the SUBSHELL it is in - The ENERGY of an orbital is defined by both the SHELL the orbital is in AND the kind of SUBSHELL it is in ARRANGEMENT OF SHELLS, SUBSHELLS, AND ORBITALS - Shells are numbered.
Cs molecular orbital diagram.
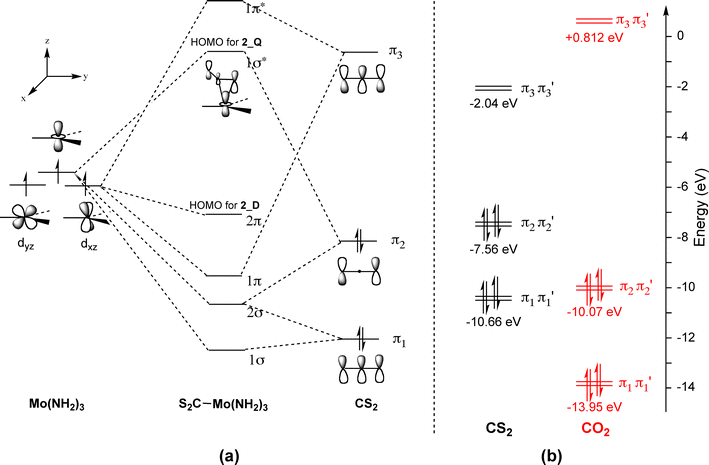
the case of CS and PN. Their ground-state electron configuration is given by (core) (50)'(60)~(70)~(2n)~ The basis specified above provides 10 occupied and 41 unoccupied (virtual) molecular orbitals. There is a large energy gap between the valence orbitals (5~, 60, 70 and
The molecular orbital diagram for #C_2# Chemistry Molecular Orbital Theory Molecular Orbital Theory. 1 Answer Stefan V. Dec 2, 2016 Here's what I got. Explanation: The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram. You need to ...
Find: Models 360. x. (June 2021) The site www.chemeddl.org is temporarily down. This is a standalone version of Models360 and links to ChemEdDL will not work. Xavier Prat-Resina is administering the current version of Models360 and its development, contact him (pratr001@umn.edu) for any question. Molecules Solids.
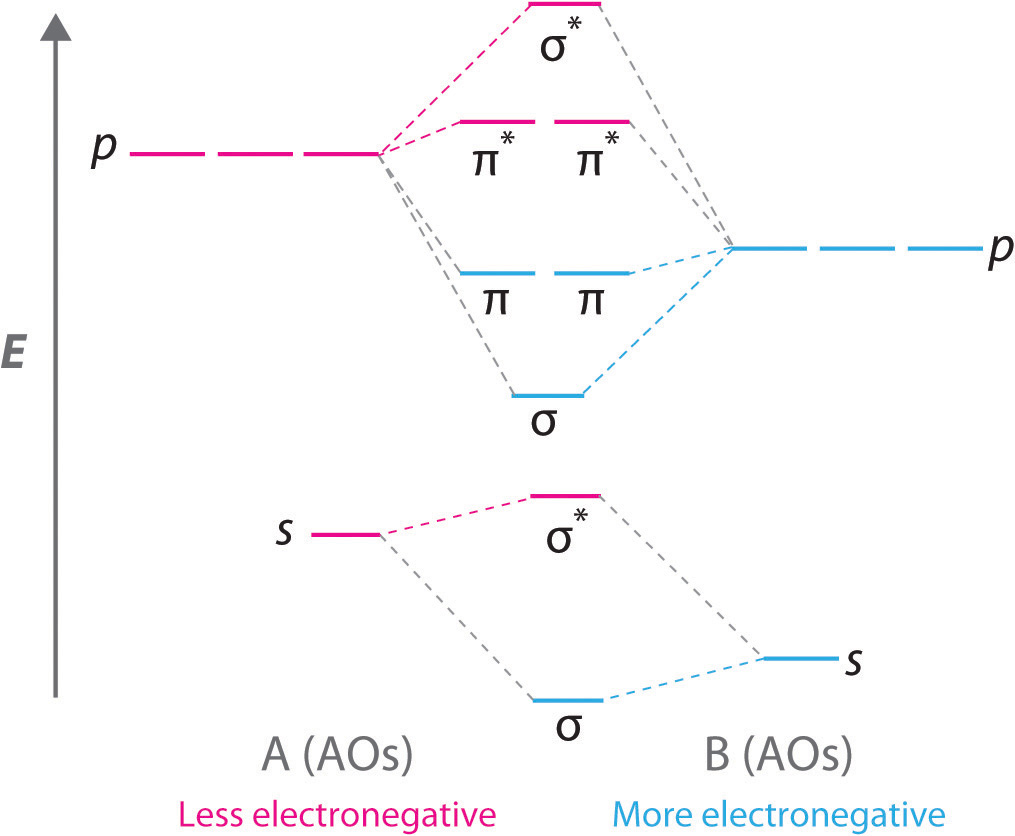
orbitals are used. So CS is like C≡O except you use 3s,3p orbitals for . the example thus ignores 1s on C and 1s,2s,2p on S Similar point, Cl uses 3s,3p and Br uses 4s,4p. Cl (right) more electronegative, so orbitals lower in energy than Br (left) orbitals are from interaction of sp hybrids, make linear C-C bonds, however the diagram
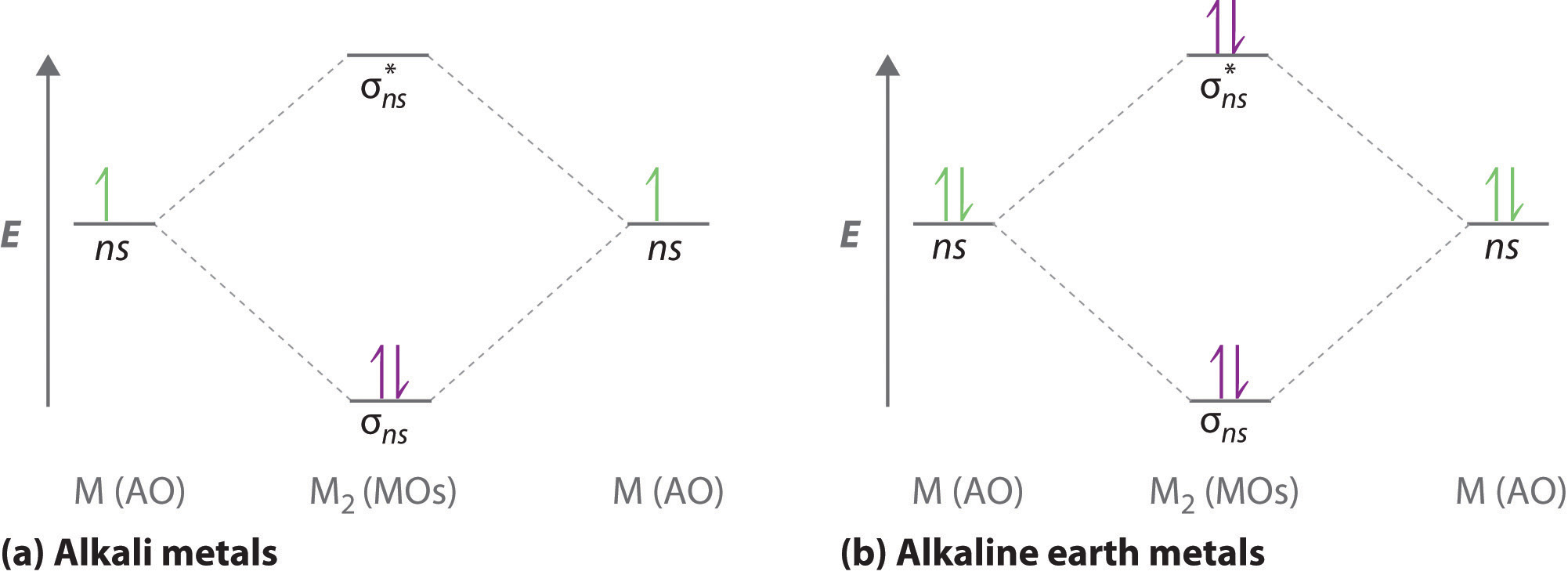
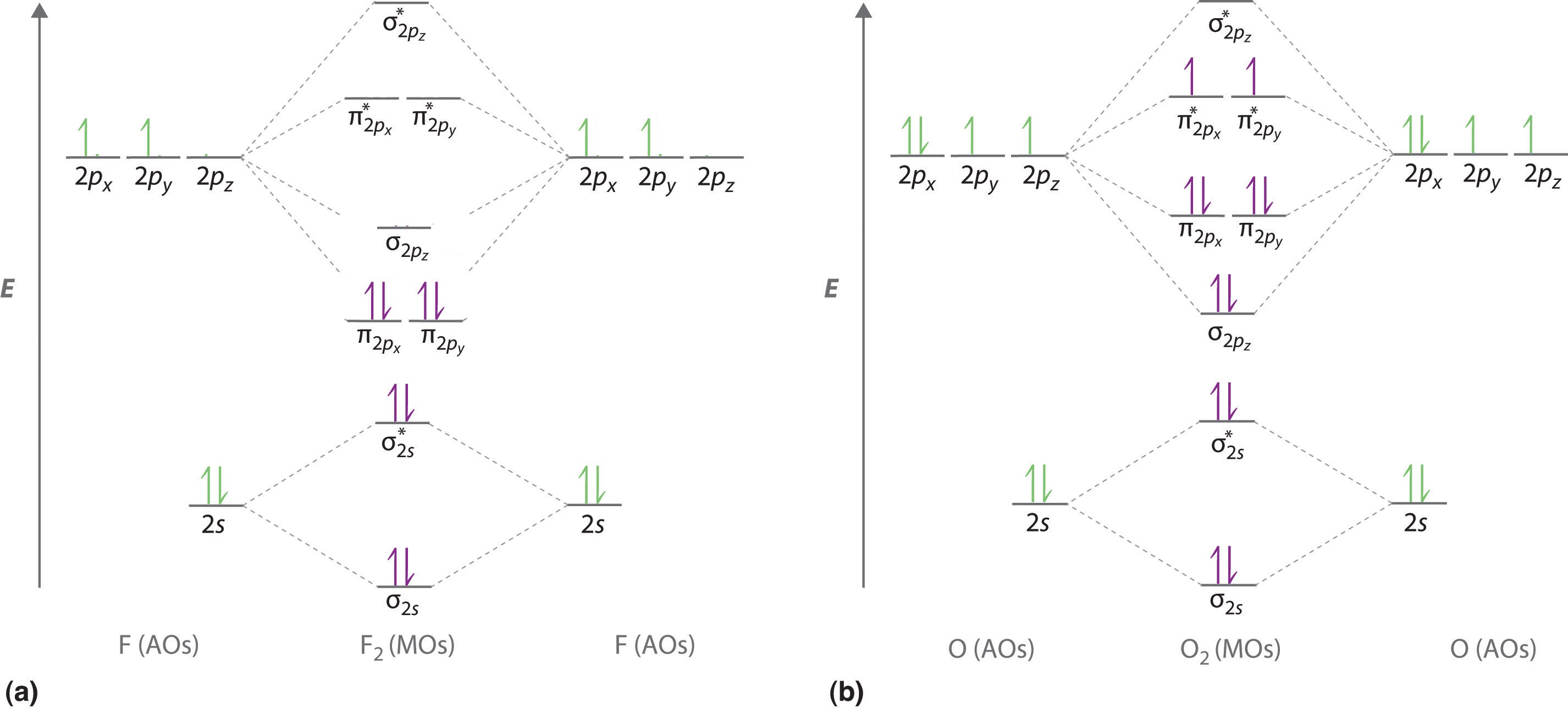
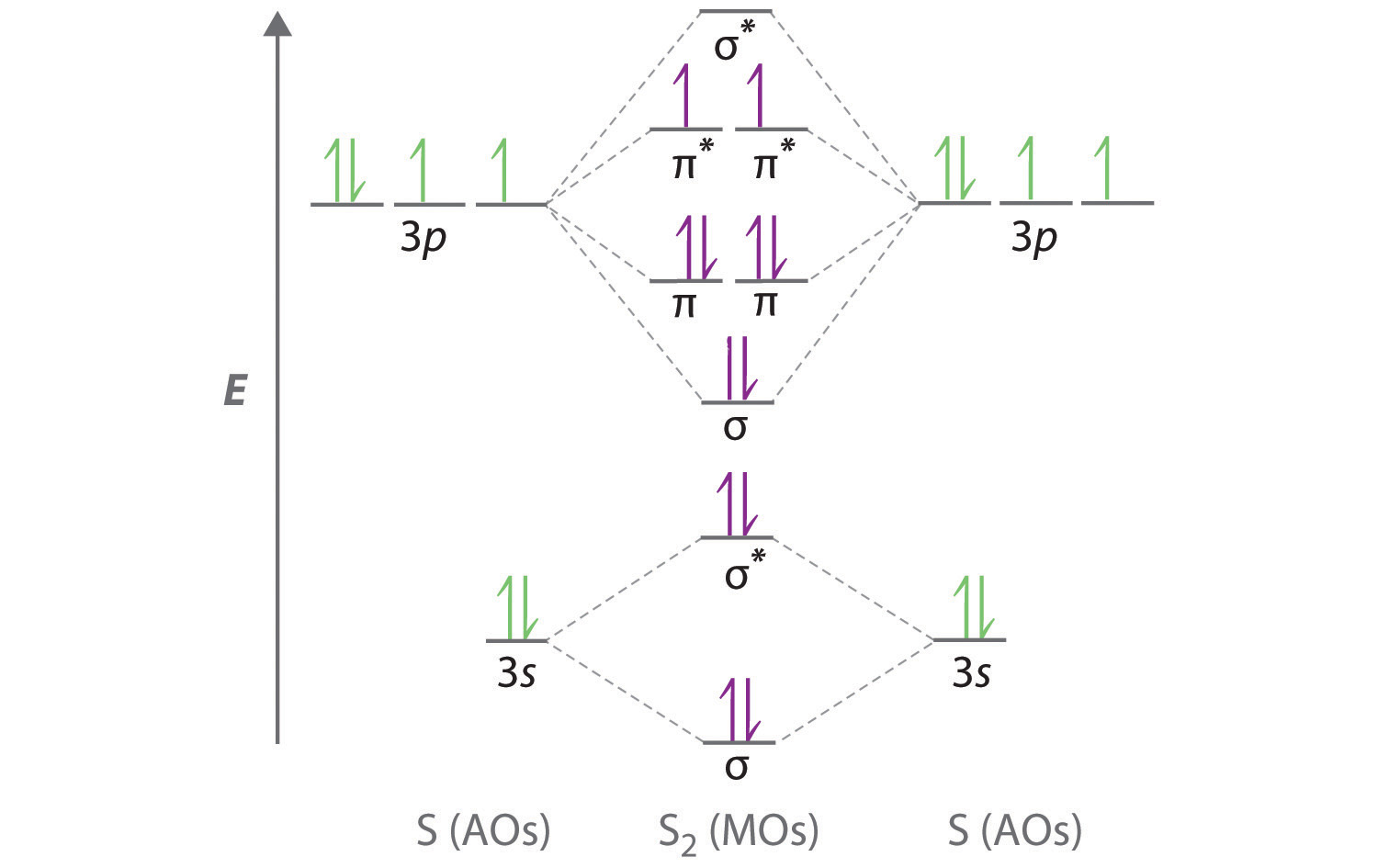
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
Let us know see how we can draw the Lewis Structure for CS2. 1. Carbon belongs to Group 4 of the periodic table. Therefore, the number of valence electrons in the Carbon atom =4. Sulfur (S) belonging to Group 6 has 6 valence electrons. CS2 has two S atoms, hence, the valence electrons in sulfur here are 6*2=12.
A partial Mo (molecular orbitals) diagram for CS is shown on the picture. a) Considering only electronegativity values, place the 3p atomic orbital for sulfur in the correct location. [Select] Answer Bank b ь b) In this diagram, which orbital is the LUMO? [Select] b) In this diagram, which orbital is the LUMO?
Molecular Orbitals --LCAO-MO Rather than pursuing the exact one-electron diatomic molecule solution further, we will use the variational method to obtain apppp proximate molecular orbitals for this problem. We will choose a trial function using a sum of one electron orbitals centered on nucleus A and one electron orbitals centered on nucleus B ...
Thus, the hydrogen bonding properties of water enhance the solubility of CO 2 over CS 2 and OCS. The LUMO orbitals for OCS and CS 2 are stable (have negative potential energy, Figure 4) and are ...
Molecular orbitals provide a great model for demonstrating molecule bonding via molecular orbital theory. Types of Molecular Orbitals. According to molecular orbital theory, some types of molecular orbitals are formed by the linear combination of atomic orbitals. These orbitals are described in more detail below.
Cs2 molecular orbital diagram. 39. The energy of σ2pz molecular orbital is greater than π2px and π2py molecular orbital s in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species : N 2, N 2+, N 2-, N ...
Molecules with Similar Molecular Orbital Diagrams Molecules and ions formed from 2 boron atoms or from 2 carbon atoms have molecular orbitals diagrams of the same sort as N2. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N2.
Oct 26, 2016 · Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular Weight: 44.08: Computed by PubChem 2.1 (PubChem release 2021.05.07) XLogP3-AA: 1.2: Computed by XLogP3 3.0 (PubChem release 2021.05.07) Hydrogen Bond Donor Count: 0: Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) Hydrogen Bond Acceptor Count: 1: Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) Rotatable Bond Count: 0
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
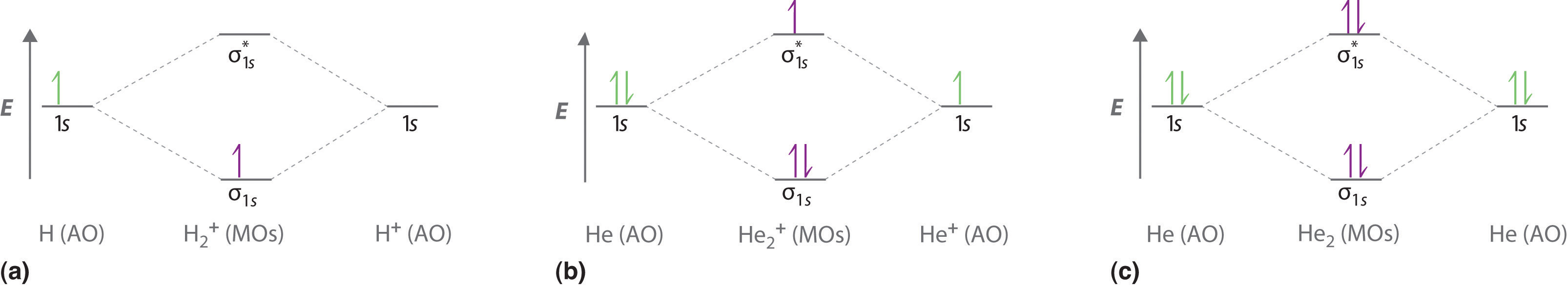
Forming Molecular Orbitals. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. Consider the H 2 molecule, for example. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1s atomic orbitals that come together to form this molecule. Another orbital is formed by subtracting one of these functions from the other, as shown in the figure below.
Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.
The molecular structure has been optimized at the B3LYP/6-31g* level of theory. Charges used for electrostatic maps are computed using the NBO method. The molecular vibrations are
This ion has a pyramidal structure with one HOH bond angle smaller than the other two, and belongs to the point group CS. a) Using a basis set consisting of a 1s orbital on each H atom and 2s, 2px, 2py and 2pz orbitals on the O atom (i.e. (sO,px,py,pz,s1,s2,s3)), construct a matrix representation.
Molecular orbitals and bond lengths found with Spartan can be used to better understand how the actual structure of a molecule or ion relates to its resonance structures. In this experiment, the valence atomic orbitals for hydrogen, carbon, nitrogen, and sulfur atoms will be calculated. Molecular orbitals for H 2, N 2, CS,
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
[Cs(18-crown-6) 2] + e ... Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Carbon Monoxide. Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals.
molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the







![Molecular orbital interaction diagram for Re[Cl(CO2)] of Cs ...](https://www.researchgate.net/publication/342215688/figure/fig5/AS:960037639970826@1605902218521/Molecular-orbital-interaction-diagram-for-ReClCO2-of-Cs-symmetry-The-populations-of.png)








0 Response to "34 cs molecular orbital diagram"
Post a Comment