35 energy diagram transition state
O exothermic O endothermic O cannot determine from the diagram Consider the mechanism. Step 1: A = B + C Step 2: C + D E Overall: A + D - B + E equilibrium slow Determine the rate law for the overall reaction, where the overall rate constant is represented as k. rate = A certain reaction has an activation energy of 56.02 kJ / mol.
May 30, 2020 — Reaction energy diagrams efficiently and effectively communicate the thermodynamics and kinetics of chemical reactions in a single diagram.
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, 430070 P. R. China. Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory, Xianhu hydrogen Valley, Foshan, 528200 China. E-mail: wujs@whut.edu.cn, msc@whut.edu.cn

Energy diagram transition state
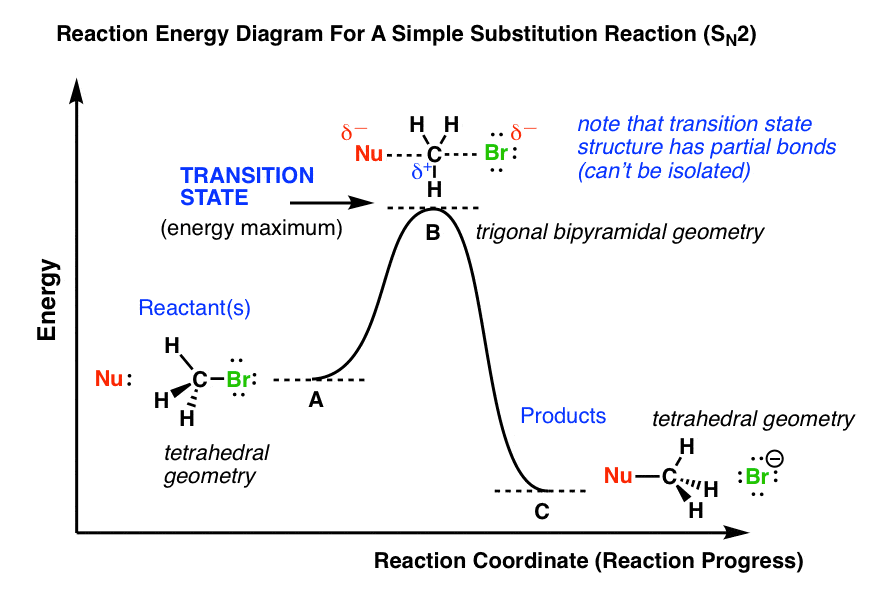
Transition states are drawn with dotted lines representing bonds that are in the process of breaking or forming, and the drawing is often enclosed by brackets. Here is a picture of a likely transition state for a substitution reaction between hydroxide and chloromethane: (6.9.3) C H 3 C l + H O − → C H 3 O H + C l −
The maximum of the energy diagram, the transition state, is characterized by a specific configuration of the atoms. Moreover, in Eyring's TST[4][5]a quite specific change of the nuclear coordinatesis responsible for crossing the maximum point, a vibration in this direction is consequently treated as a translation.
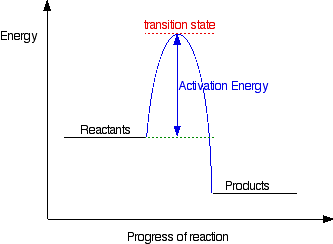
Carefully complete the energy diagram by drawing a curve that accurately shows the progress of the reaction, beginning at the reactants, moving through the transition state, and ending at the final products. When adding the products to the diagram, be sure to use an energy level that is appropriate relative to the energy level of the reactants.
Energy diagram transition state.
At the transition state (TS) and further along the reaction pathway, the peaks at the Fermi level are greater than 0.1 eV. The corresponding rate of electron transfer is approximately 10 14 s −1,...
Since hardware scheduler feedback is computed before performing the power budgeting based in the energy performance preferences, this ensures that even if a thread is scheduled in/out by the operation system the feedback will not change, thus providing the present power/thermal state of the system irrespective of the thread scheduled.
Transition state theory (TST) explains the reaction rates of elementary chemical reactions.The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.. TST is used primarily to understand qualitatively how chemical reactions take place.
Influence of the hyperfine and an external magnetic field B on the energy-level diagram (in reference to the unperturbed energy) for the state Sr 87 | (5 s 6 p) P 1 1 〉. Presented here is an approximation in that F is treated as a good quantum number which is valid only for small B. To convert to joule, multiply by the Planck constant h ...
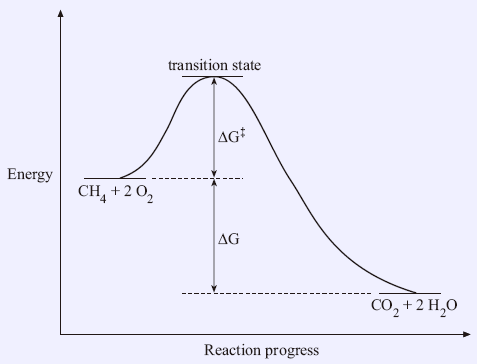
For most energy diagrams, the energy values are orientative, made up in order to explain the concepts. ... And what happens when the transition state of the rate-limiting step of a reaction is lower in energy? That the activation barrier of the whole process is much lower! This is basically the role of FeBr 3 (the catalyst) of this reaction ...
Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products .
(D) Energy diagram of electron transition between hydrogen and oxygen. In addition, the schematic diagram of possible physical processes of electrons transitions and the associated photon emission, also known as Wang transition , when two atoms are close to each other (E to H) (see text for details).
The Questions and Answers of For a diatomic molecule AB, the energy for the rotational transition from J = 0 to J = 1 state is 3.9 cm-1. The energy for rotational transition from J = 3 to J = 4 state would be (a) 3,9 cm-1 (b) 7.8 cm-1 (15.6 cm-1 (d) 11.7 cm-1 ? are solved by group of students and teacher of Physics, which is also the largest ...
The oxygen reduction reaction (ORR) is the key bottleneck in the performance of low-temperature fuel cells 1,2,3,4.The most active catalysts so far for the reaction are based on Pt group metals ...
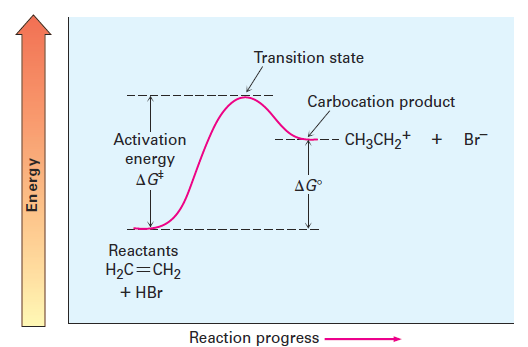
May 15, 2017 — Figure 1.1 An energy diagram for the first step in the reaction of ethylene with HBr. The energy difference between reactants and the transition ...
Nov 3, 2010 — A transition state is a very short-lived configuration of atoms at a local energy maximum in a reaction-energy diagram (aka reaction ...
15/08/2020 · A Jablonski diagram is basically an energy diagram, arranged with energy on a vertical axis. The energy levels can be quantitatively denoted, but most of these diagrams use energy levels schematically. The rest of the diagram is arranged into columns. Every column usually represents a specific spin multiplicity for a particular species.
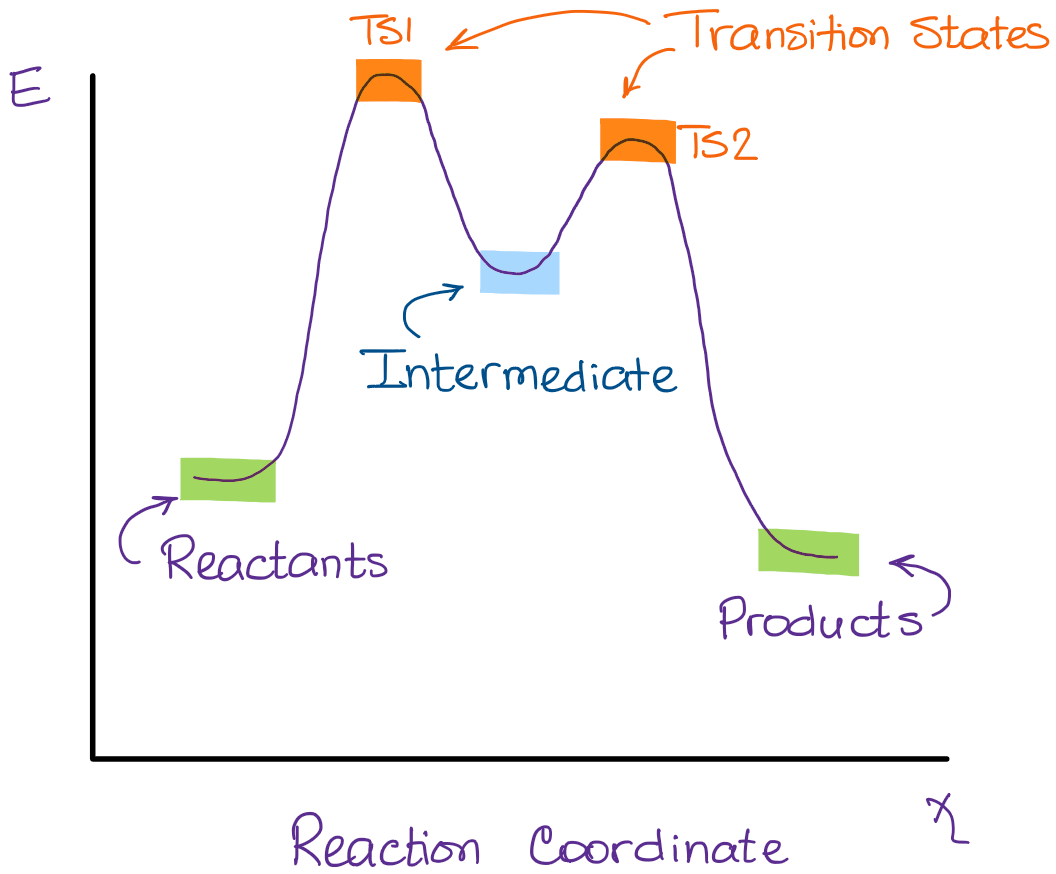
3.1. Reaction of Ethylene with ReO 3 F. The relative energies of the main stationary points (reactants, transition states, intermediates, and products) and some optimized structures involved in the reaction between ethylene and ReO 3 F are shown in Figures 1(a) and 1(b), respectively.The singlet ReO 3 F species has its three Re = O bond lengths at 1.704 Å while the Re-F bond equals 1.804 Å.
The inset shows the shape and strain distribution of this mode calculated by FEM. (d) PLE spectrum of the Y 1 − Z 1 transition of the Er ions embedded in the resonator. The inset shows the energy diagram of this transition. (e) Energy decay of the excited state of Er ions and the mechanical mode.
The potential energy diagram was adapted with permission from ref. 2, ... on the other hand, has a large energy span between the most stable state ... and the highest transition state, ...
Also during the transition from the solid to the liquid state, a sudden change of the binding energy occurs. While the molecules in the solid state are firmly bound to a specific location due to the great binding forces, the molecules in the liquid state can move relatively freely due to the weak binding forces.
(b) DAPS measurement for thr same QD but with a 33-GHz rf tone applied to a nearby gate. Some two-photon transitions are also visible, whose positions correspond well with the dc and rf resonances expected from the energy-level diagram depicted. The faint transition on the far right potentially corresponds to a two-photon resonance.
What is the energy diagram for the rotational interconversion of s-cis to s-trans 1.3-butadiene? What is the MO scheme for the transition state of thi … s bond rotation? What happens if you excite ethene, acetone or O2 (by looking at the MO schemes!!)? Rationalise the reactivity patterns of the corresponding excited sp …
such processes are impossible to observe. The energy scale was calibrated by using the known energy of the diagram transition to the ('P;3uJ2 doubly ionized state at 366.9 eV (Siegbahn et a1 1971). In figure 1 we show an overview of the Auger spectrum of N2 as obtained by excitation with monochromatic x-ray radiation. The figure shows
Ben rewrites the state transition diagram as a state transition table (Table 3.1), which indicates, for each state and input, what the next state, S′, should be. Note that the table uses don’t care symbols (X) whenever the next state does not depend on a particular input. Also, note that Reset is omitted from the table.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are …
Here, we report a new state-diagram for aqueous solutions based on concentration-dependent glass-transition temperatures of concentrated and ice freeze-concentrated solutions. Different from the eq...
In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This …
In state transition diagram the states are shown in boxed texts, and the transition is represented by arrows. It is also called State Chart or Graph. It is useful in identifying valid transitions. In state transition table all the states are listed on the left side, and the events are described on the top.
Transition state diagram / reaction coordinate diagram: Plot of free energy versus the reaction coordinate.Transition State Diagram (Symetrical)! Substrate! Product! Transition ... Enzyme s catalyze reaction s by preferentially binding the transition state An enzyme may binds the transition state of the A reaction coordinate diagram comparing an uncatalyzed reaction with an enzyme-catalyzed ...
The energy transfer pathway from an organic antenna to the lanthanide ion usually follows the sensitization from the lowest singlet state, S 1, to T 1 and then to Ln 3+ 10, 11. Intersystem crossing...
The energy transition is a major factor in the rise of renewables, but solar's growth is partly due to how cheap it has become over time. Solar energy costs have fallen exponentially over the last decade, and it's now the cheapest source of new energy generation.
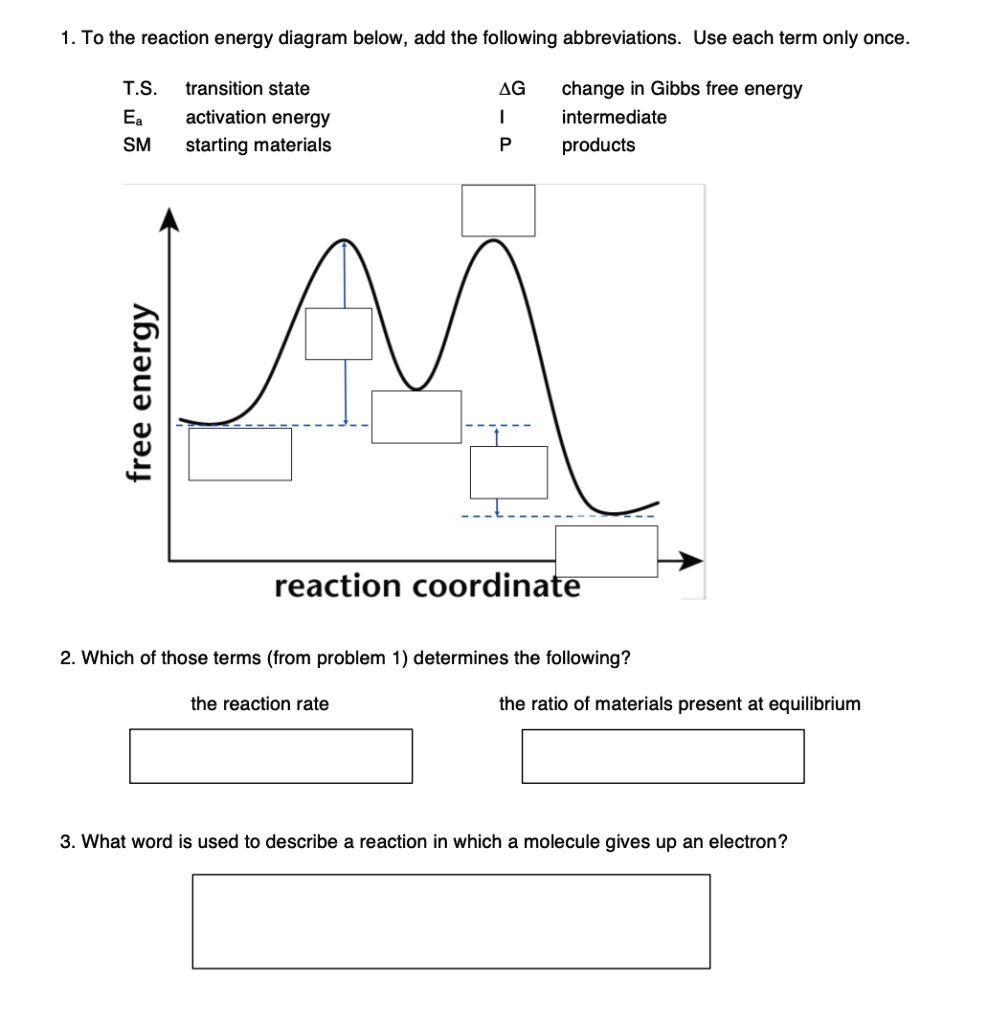
Carefully complete the energy diagram by drawing a curve that accurately shows the progress of the reaction, beginning at the reactants, moving through the transition state, and ending at the final products. When adding the products to the diagram, be sure to use an energy level that is appropriate relative to the energy level of the reactants.
Orbital state energy level: atom/ion with nucleus + one electron. Assume there is one electron in a given atomic orbital in a hydrogen-like atom (ion). The energy of its state is mainly determined by the electrostatic interaction of the (negative) electron with the (positive) nucleus.
When plotted in a stress-energy phase diagram, the two responses are observed to form separate lines that intersect the static hcp-bcc phase transition and incipient melt. The results indicate that the hcp-bcc transition occurs on the Hugoniot at 28.4 GPa and place the hcp-bcc-liquid triple point \ensuremath{\sim}20 GPa.
A diagram showing the various kinds of electronic excitation that may occur in organic molecules is shown on the left. Of the six transitions outlined, only the two lowest energy ones (left-most, colored blue) are achieved by the energies available in the 200 to 800 nm spectrum.
Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not …
In a multi-step reaction, each step has a transition state and corresponding activation energy. The transition states of such reactions are punctuated with ...
The branching ratios of photoemission decays from the excited 1 − states to the ground state (green curve), the 2 1 + state (red curve), and the 2 2 + state (blue curve) in Sn 140. The horizontal axis is the excitation energy of the 1 − states. The neutron separation energy S 1 n = 2.59 MeV is indicated by the dashed line.
Reaction coordinate diagrams — Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B.
21/11/2010 · The ionization energy of an atom is the energy required to remove the electron completely from the atom.(transition from ground state n = 0 to infinity n = ∞). For hydrogen, the ionization energy = 13.6eV; When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
This transition state has a geometry with the H-H bond distance shorter than 1.1 Å and the two O-H bond lengths around 1.8 Å. The calculated energies and average bond distances of vibrational...


















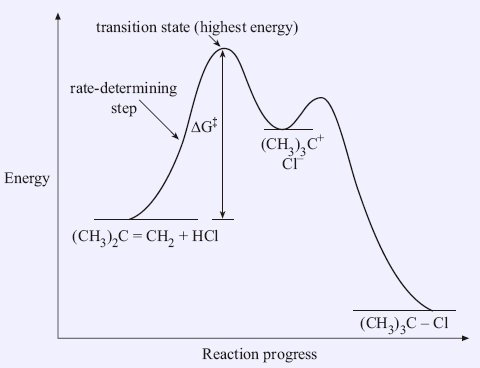





0 Response to "35 energy diagram transition state"
Post a Comment