35 full orbital diagram for ne
Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: ... (Full Chart Inside) Atomic Radius of All the Elements (Complete Chart Inside) Electron Configuration of All Elements (Full Chart Inside) ...
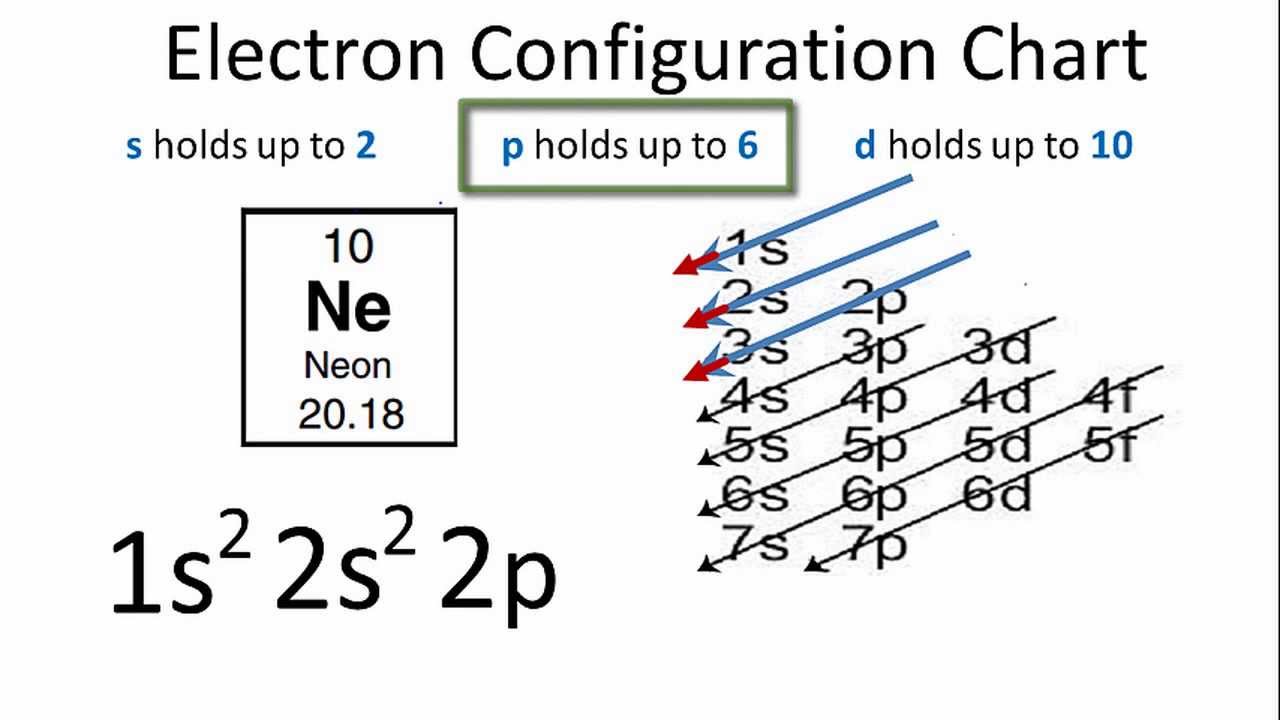
21 Jan 2021 — The electron configuration of the Ne can write as 1s22s22p6. It simply depicts the theory that the first two orbital the 1s and the 2s are ...
Neon (Ne) has an atomic mass of 10. Find out about its chemical and physical ... Electron Configuration, [He] 2s2 2p6. 1s2 2s2 2p6. Orbital Diagram.
Full orbital diagram for ne
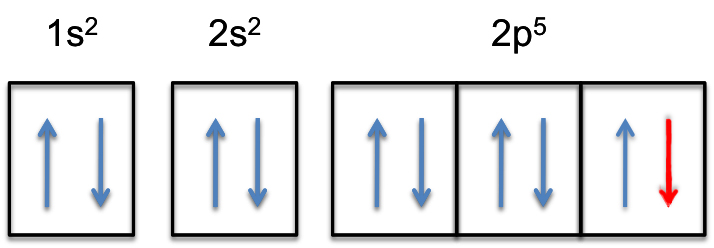
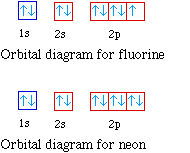
Neon (Ne) Electron Configuration With Full Orbital Diagram Electron Configuration of all elements Group-18 Electron Configuration Neon (Ne) Electron Configuration with Full Orbital Diagram Neon electron configuration is 1s 2 2s 2 2p 6. The symbol for neon is ‘Ne’ and it is an inert element.
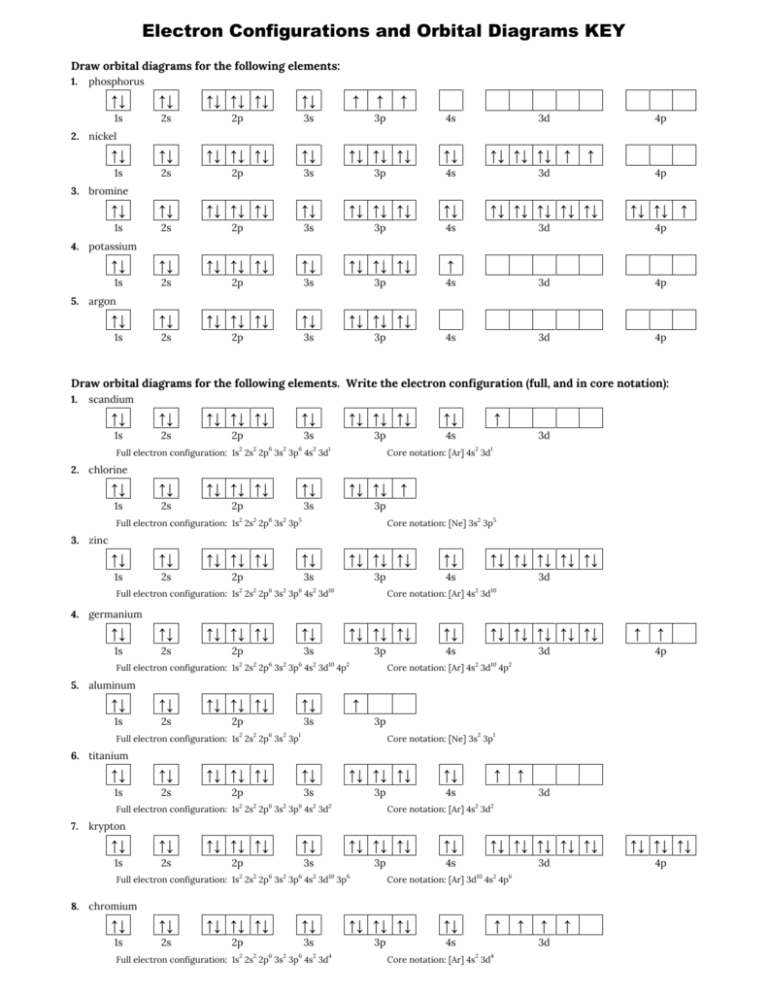
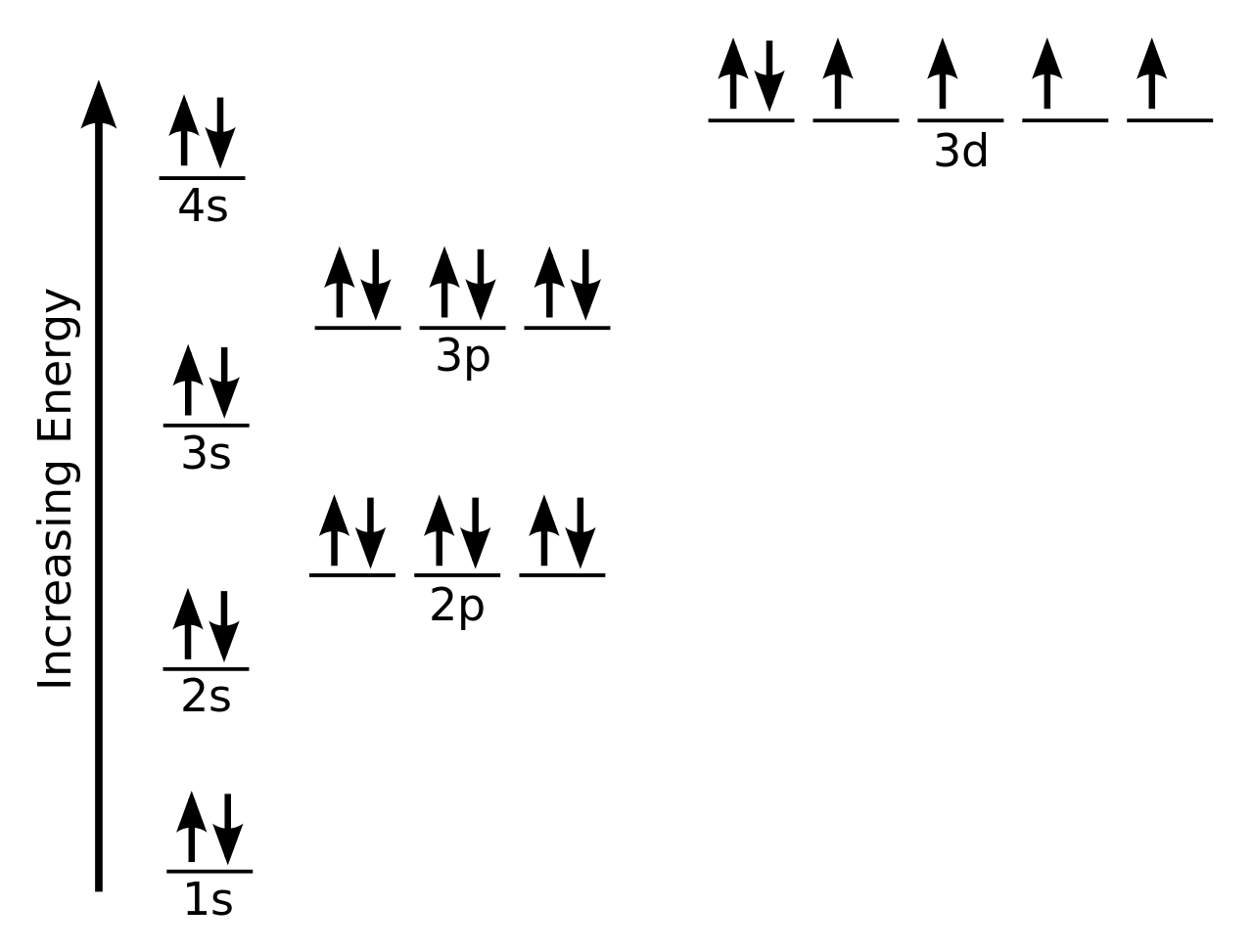
Answer to Draw the full orbital filling diagram for Sulfur, make sure you show the energy relationship between orbitals and label. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each.
Full orbital diagram for ne. 24-09-2019 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you ne ed to know the number of protons, ne utrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen.
Full orbital diagram for ne.
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
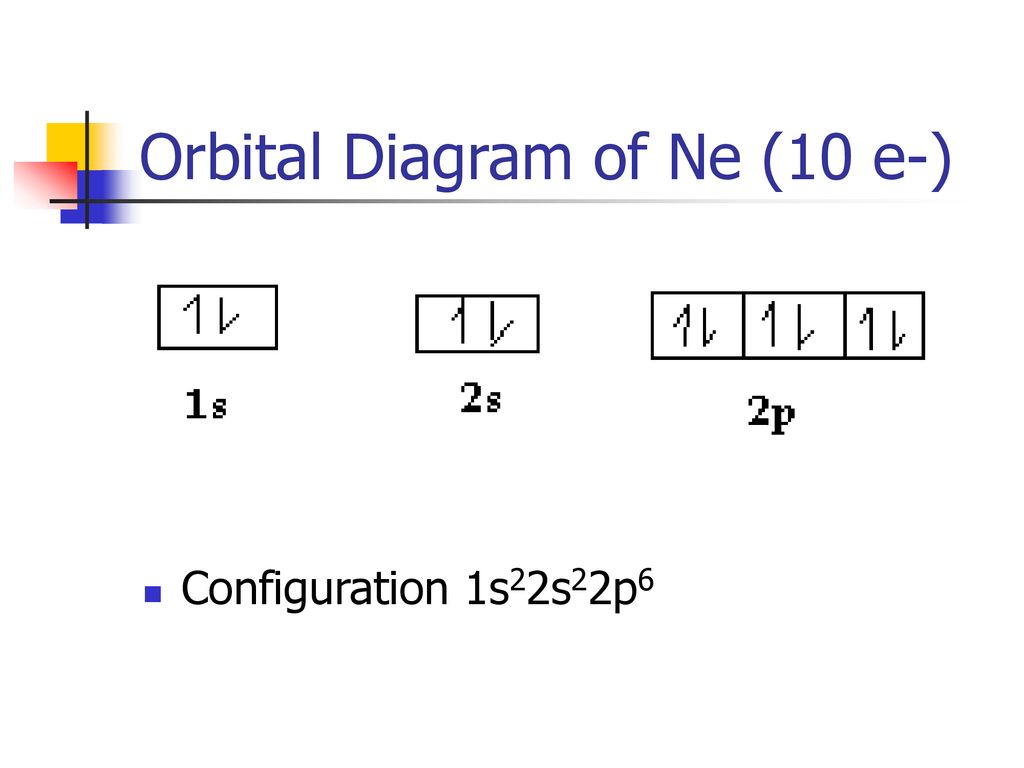
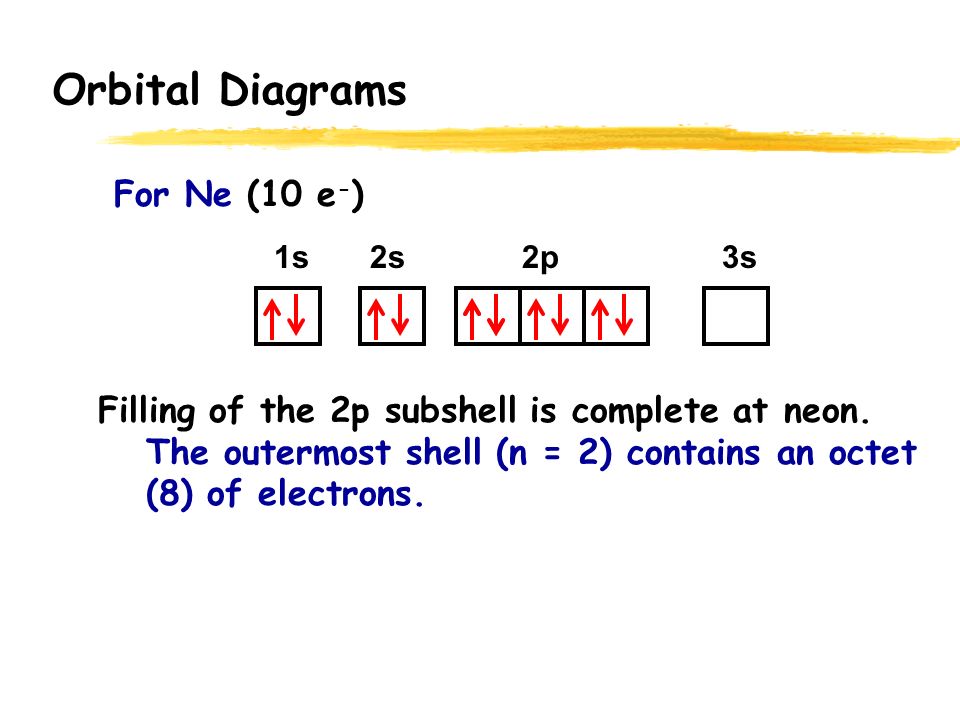
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital.
8 May 2021 — Full Electron Configuration, Nobel Gas Shorthand. Neon, Z = 10, Ne: 1s2 2s2 2p6, Ne: [He] 2s2 2p6. Sodium, Z = 11. Na: 1s2 2s2 2p6 3s1.
Sodium(Na) electron configuration with full orbital diagram. Sodium is the eleventh element in the periodic table and the 3rd element in group-1. The atomic number of sodium is 11 and its symbol is ‘Na’. ... (Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, ...
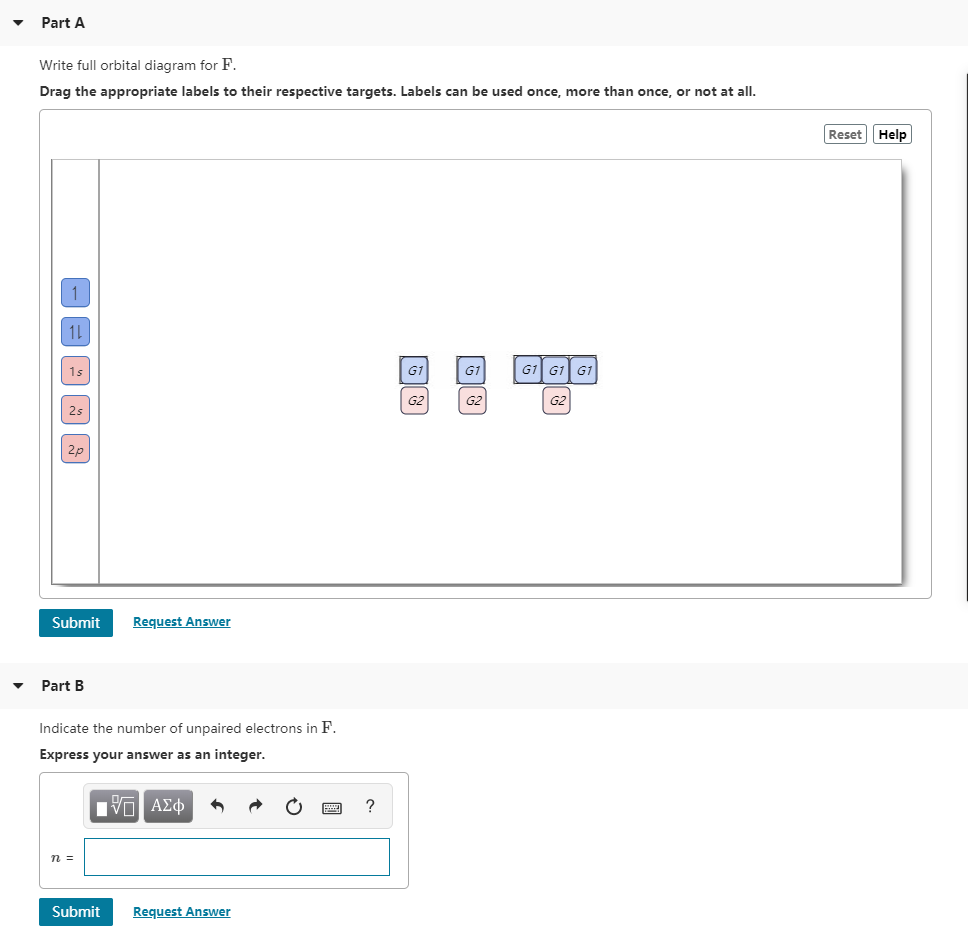
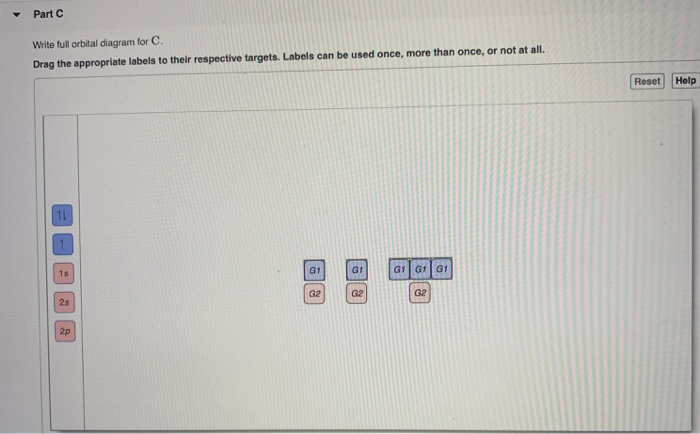
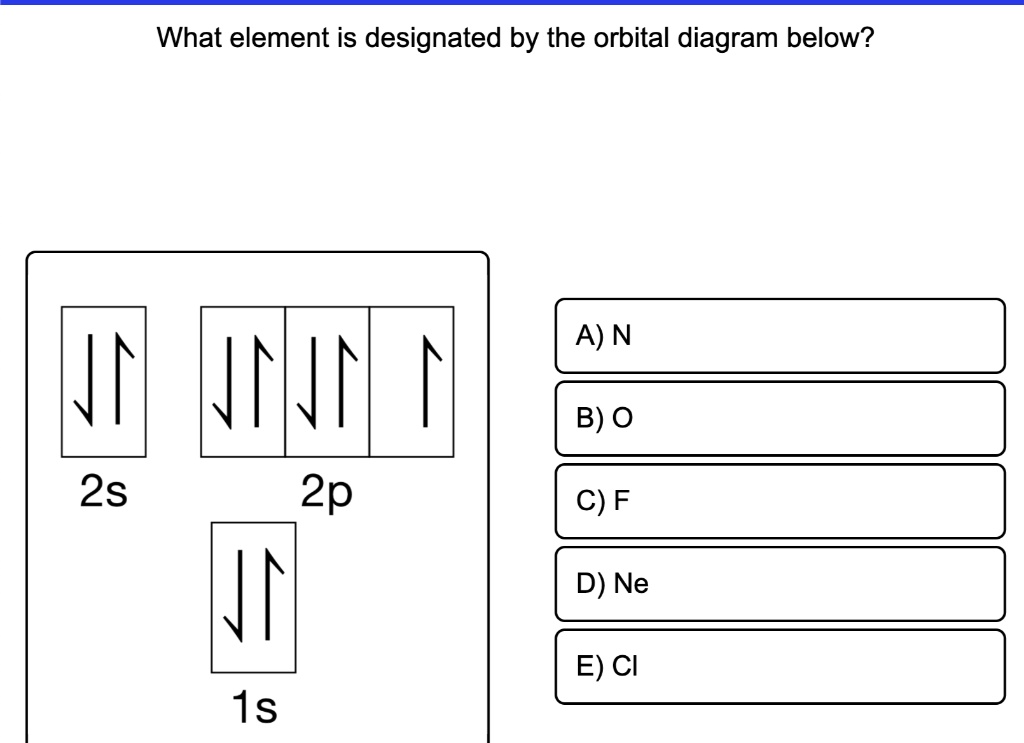
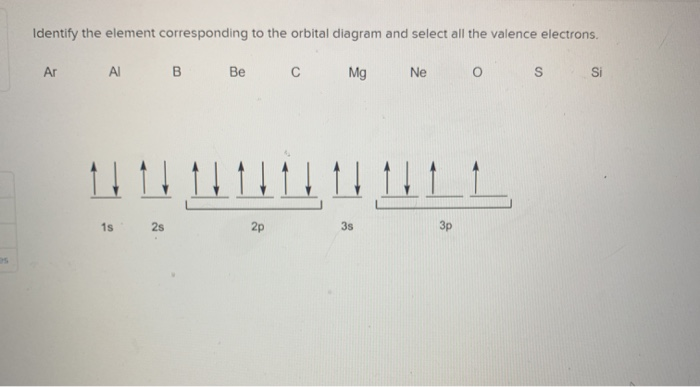
Write a full orbital diagram for each element. a. N b. F c. Mg d. Al. a. 1s^2 2s^2 2p^3 (p boxes only half filled) b. [He] 2s^2 2p^5 (p boxes missing only 1) c. [Ne] 3s^2 (3s boxes full) d. [Ne] 3s^2 3p^1 (3p box missing 5) 46. Use the periodic table to determine the element corresponding to each electron configuration. a. [Ar] 4s^2 3d^10 4p^6
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
One can easily show the electronic arrangement in different orbitals of an element, using the basic concept of orbital diagram. This requires the knowledge of element's atomic number. Answer and...
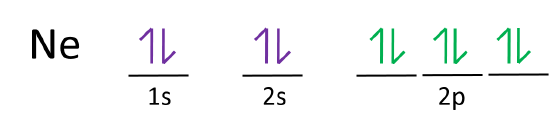




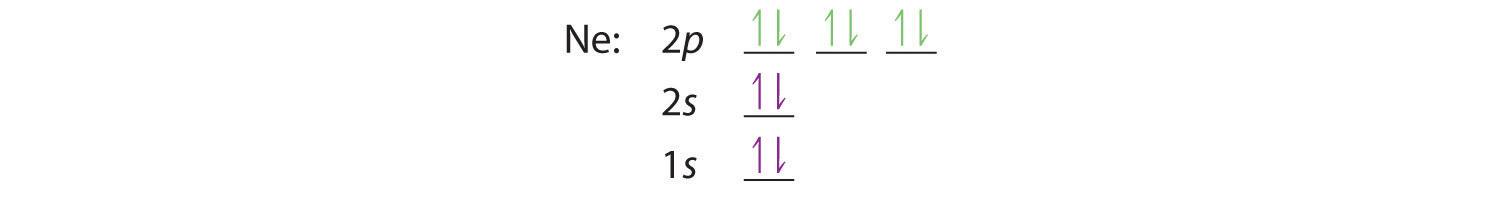




![Unsur yang mempunyai diagram orbital: [Ne] 4s 3p adalah ....](https://coln-prd-sg-s3-ads-pub.s3.ap-southeast-1.amazonaws.com/images/questions/K0060631P016.png)








0 Response to "35 full orbital diagram for ne"
Post a Comment