35 write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):
Write A Balanced Equation and Draw An Enthalpy Diagram for (select if Exothermic or Endothermic): question write a balanced equation and draw an enthalpy answer to write a balanced equation and draw an enthalpy diagram for select if exothermic or endothermic [ select ] ["endotherm write a balance and draw an approximate enthalpy diagram write a balance and draw an approximate enthalpy diagram ...
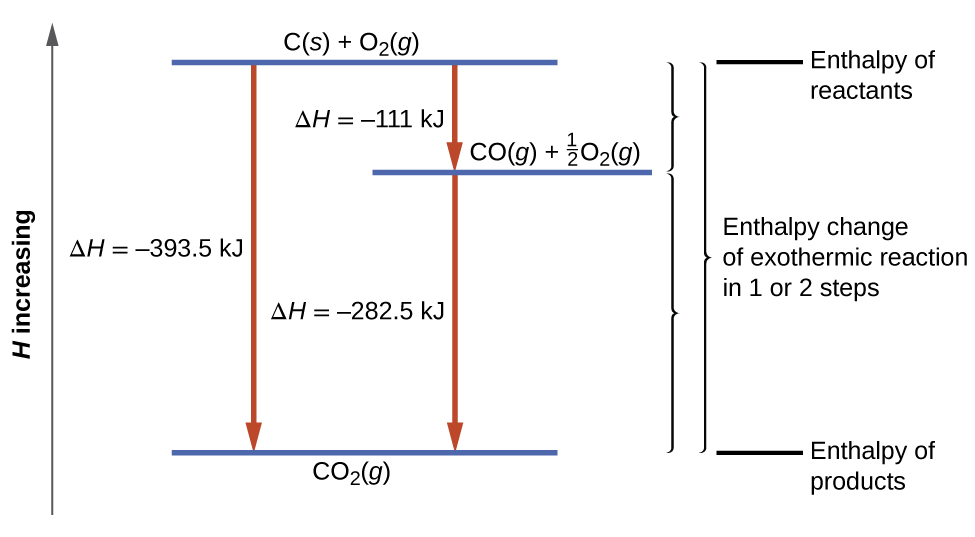
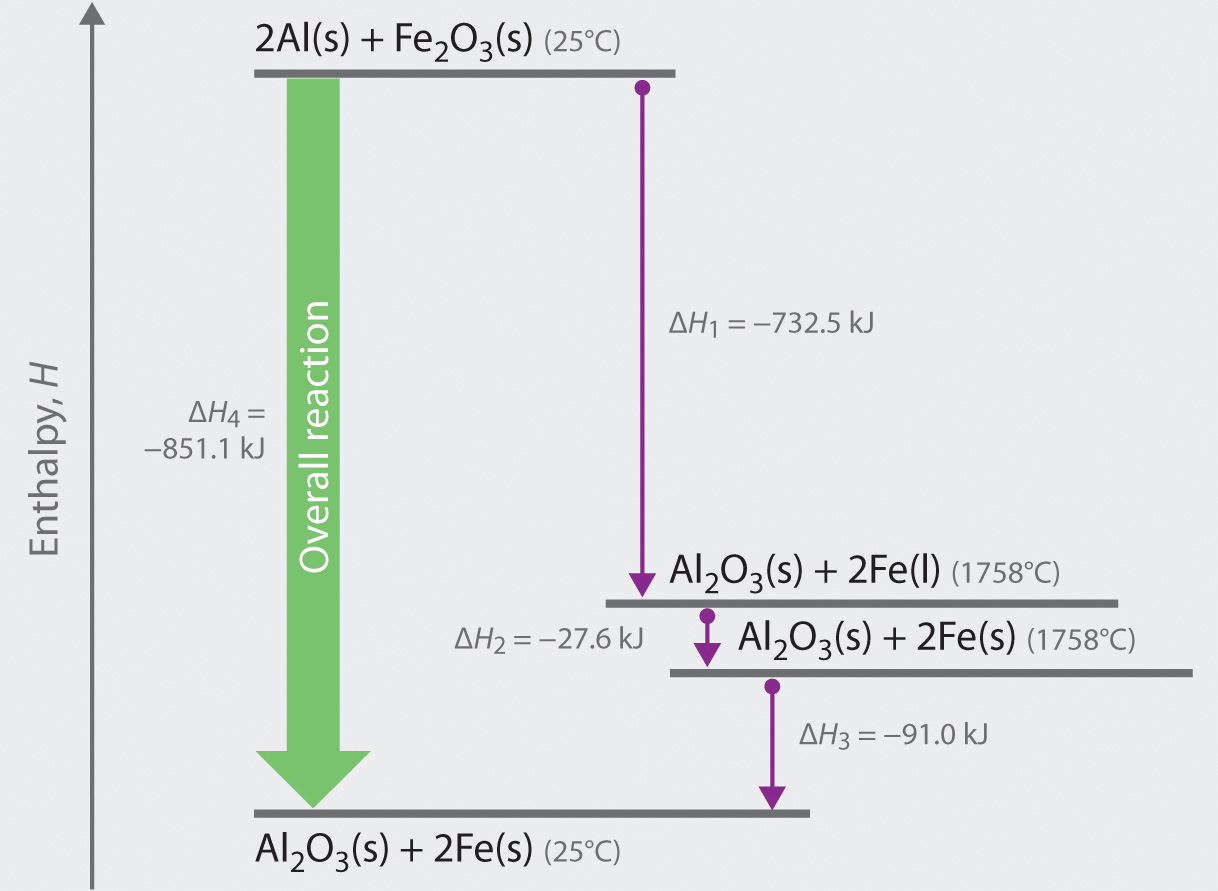
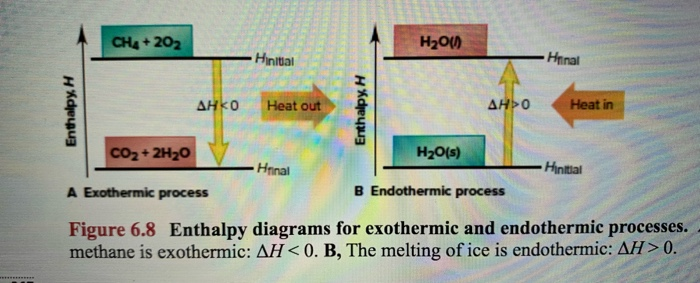
If heat is given off, it is called an exothermic reaction. If heat is absorbed, it is called endothermic reaction. In this chapter we will learn how to calculate the enthalpy change (heat change) when methane (a gas) is formed using carbon graphite and hydrogen. Hess's law. Hess's law is used to determine the enthalpy change.
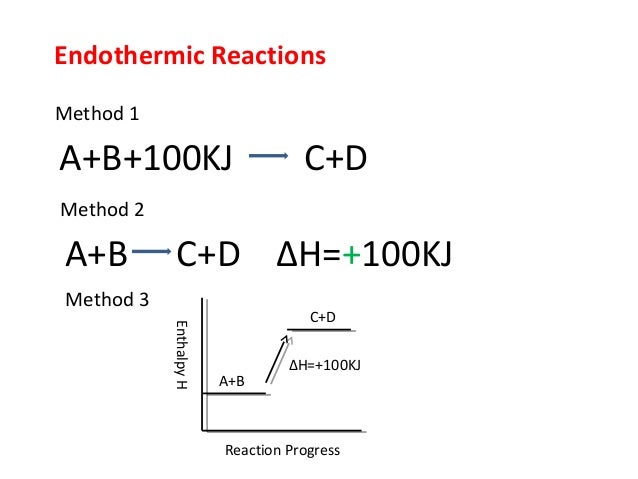
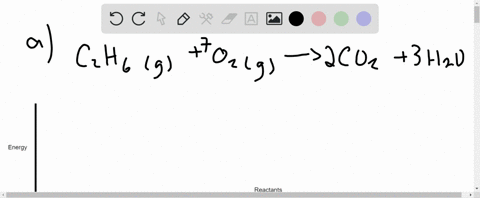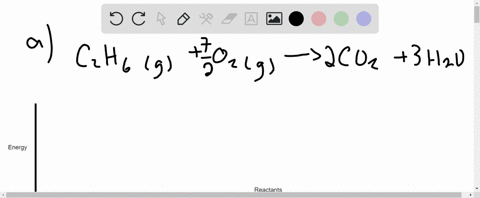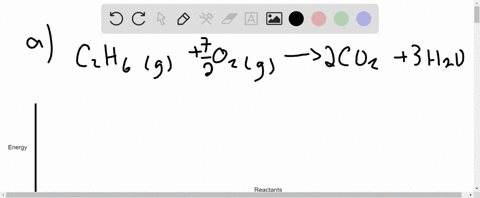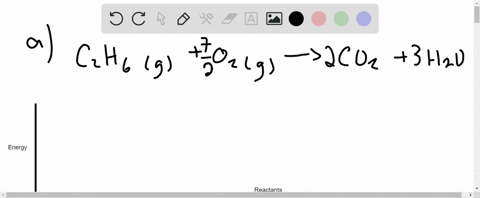
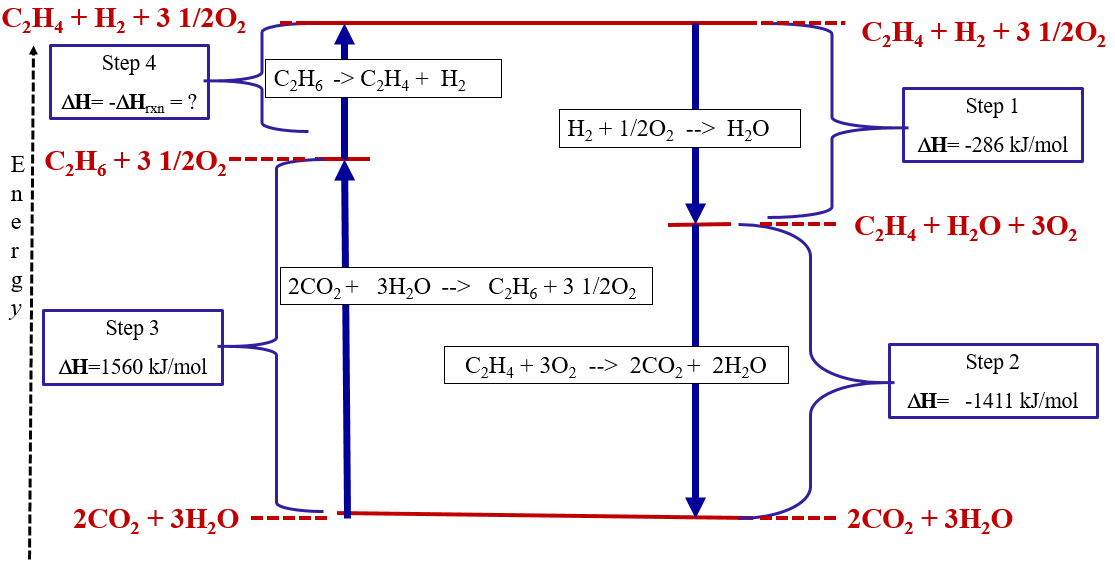
Q. Draw an enthalpy diagram for a general endothermic reaction; label the axis, reactants, products, and ΔH with its sign. Q. When 2 moles of C2H6 (g) react with O2 (g) to form CO2 (g) and H2O (g) according to the following equation, 2.86 x 103 kJ of energy are evolved.2C2H6...
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):
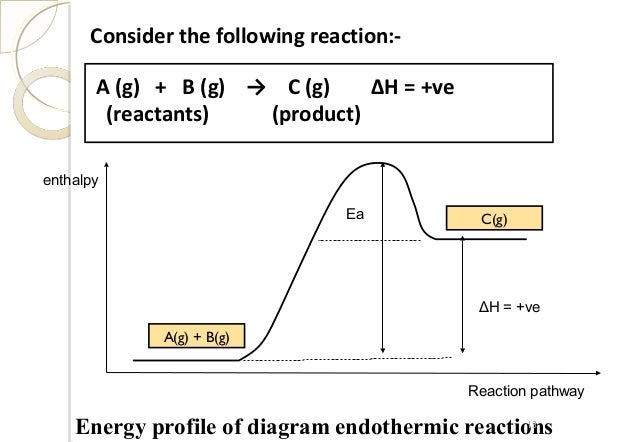
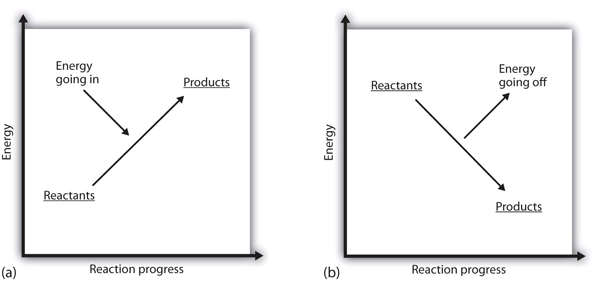
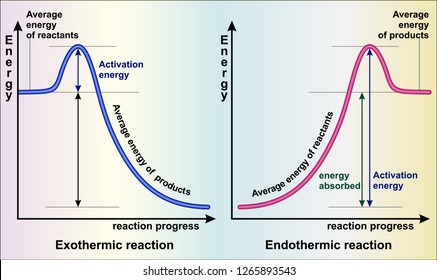
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
This photo about: Write A Balanced Equation and Draw An Enthalpy Diagram for (select if Exothermic or Endothermic):, entitled as Fibers Free Full Text Write A Balanced Equation And Draw An Enthalpy Diagram For (select If Exothermic Or Endothermic): - also describes Fibers Free Full Text and labeled as: write a killer cover letter,write a letter to the principal to request of certificate,write ...
Write an equation for the reaction. (d) Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. (Assume the iron oxide contains Fe 3+ ions.) Fill in the blank with a single chemical formula for a covalent compound that will balance the equation:
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):.
6. Given either the initial and final temperature measurements of a solution or the sign of the ∆H rxn, identify if a reaction is endothermic or exothermic. 7. Given the change in enthalpy for a reaction, the amounts of reactants, and a balanced chemical equation, calculate the heat exchanged for a reaction.
The general equation for an exothermic reaction is: Reactants → Products + Energy. If the energy produced in an exothermic reaction is released as heat, it results in a rise in temperature. As a result, the products are likely to be warmer than the reactants. How do you know if an equation is endothermic or exothermic?
This problem has been solved! Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): [ Select ] ["endothermic", "exothermic"] combustion of one mole of methane. [ Select ] ["endothermic", "exothermic"] vaporization of liquid alcohol.
Since enthalpy is measured in kJ/mol, the coefficients in the equation represent the amount in moles of each reacting substance. e.g. 2H2 (g) + O2 (g) ==> 2H2O (l) delta H = -572 kJ/mol Can be read as: when 2 moles of hydrogen react with 1 mole of oxygen, 2 moles of water form and 572 kJ of energy is produced.
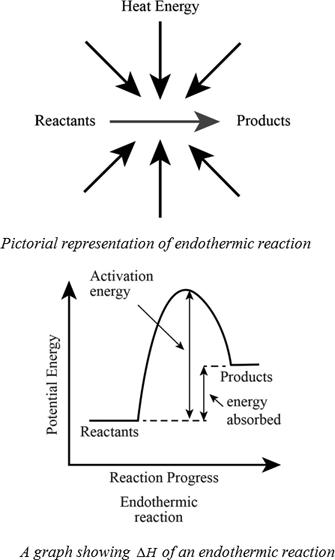
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical processes can be endothermic as well - Ice cubes absorb heat energy from their surroundings and melt to form ...
Endothermic and Exothermic: During a chemical reaction, energy is either transferred to or from its surroundings. Reactions which involve heat energy being released into the surrounding environment are called exothermic reactions. By contrast, endothermic reactions draw heat energy from their surroundings. This causes the temperature of the ...
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.
Always to the right of the arrow in a chemical equation. (the arrow is pointing at the products) In exothermic reactions, there is more energy in the reactants than in the products. In endothermic reactions, there is less energy in the reactants than in the products.
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): [ Select ] ["endothermic", "exothermic"] combustion of one mole of methane [ Select ] ["endothermic", "exothermic"] vaporization of liquid alcohol
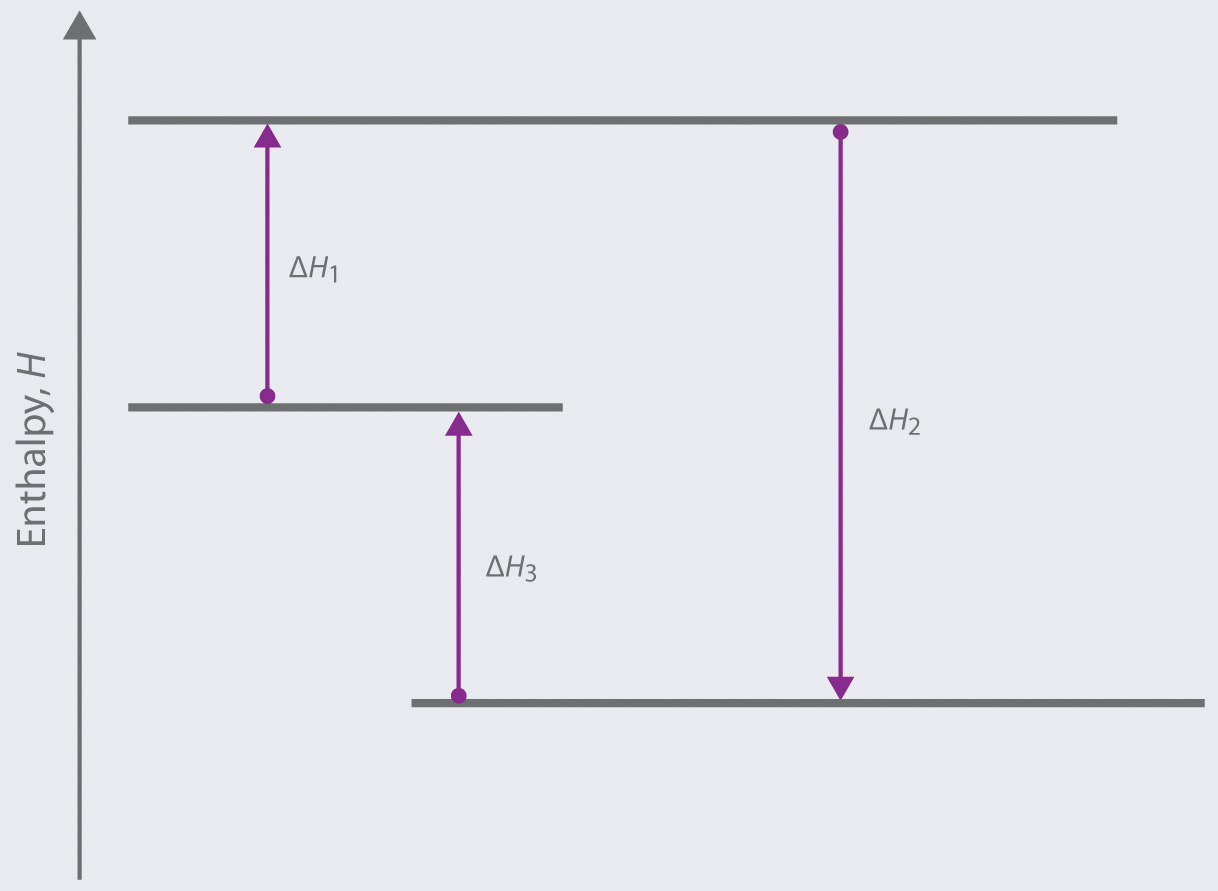
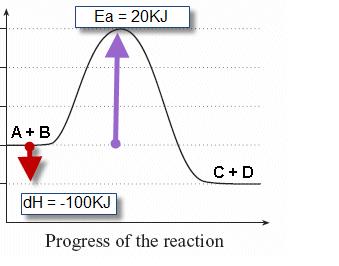
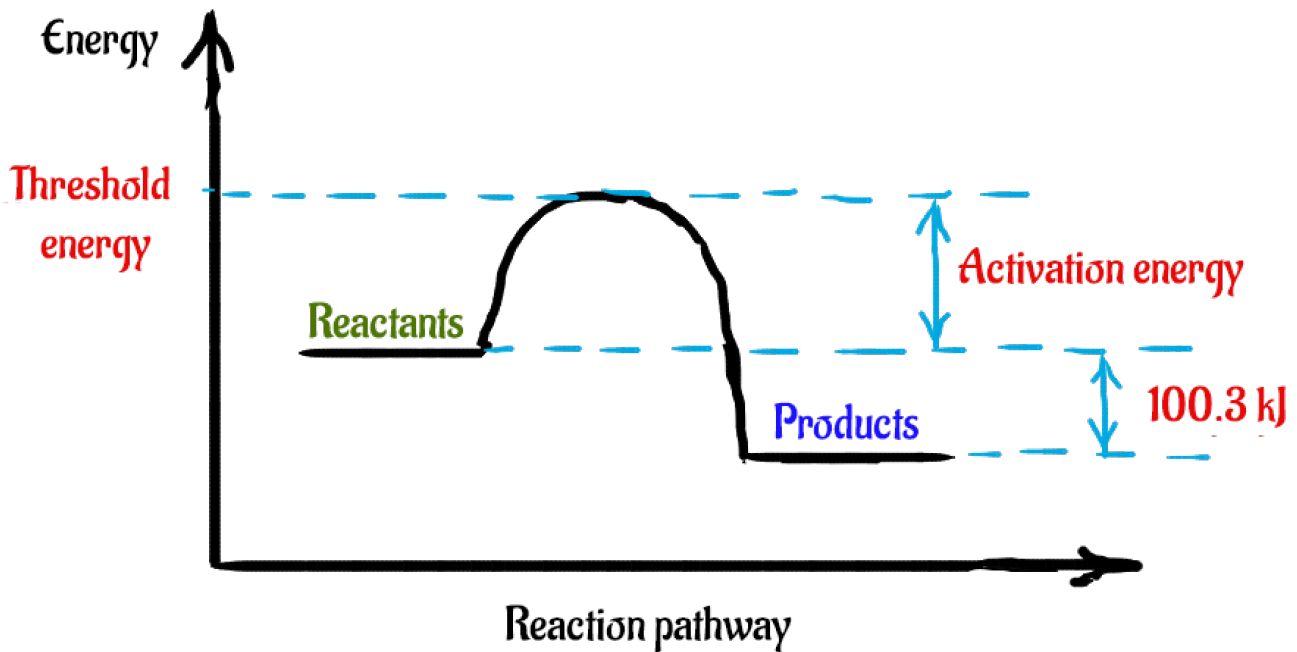
Identify whether the reaction is exothermic or endothermic: 2: Draw and label the energy axis: 3: Draw the energy level for the reactants and products: 4: Draw an arrow from the reactants level to the products level: 5: Write in the reactants and products based on the balanced equation: 6: Label ΔH as positive or negative
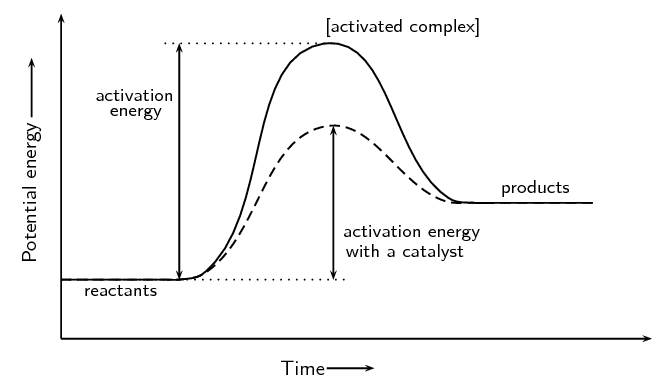
Write the balanced thermochemical equation for the combustion of methane gas, CH 4(g). b. Draw a potential energy diagram that would reasonably represent this combustion reaction. Indicate the Δ H comb and a molecular structure that could represent an activated complex in this potential energy diagram. What Is Required?
Endothermic Processes. These examples could be written as chemical reactions, but are more generally considered to be endothermic or heat-absorbing processes: Melting ice cubes. Melting solid salts. Evaporating liquid water. Converting frost to water vapor (melting, boiling, and evaporation, in general, are endothermic processes.
Solution for Draw an enthalpy diagram for the following reaction and state whether the reaction is endothermic or exothermic. NaOH + HCl → NaCl + H2O +58 kJ close. Start your trial now! First week only $4 ... Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, wri...
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): How can you tell if a balanced equation is endothermic or exothermic? So if the sum of the enthalpies of the reactants is greater than the products, the reaction will be exothermic.
44 Given the balanced equation representing a reaction: Which statement is true about energy in this reaction? (1) The reaction is exothermic because it releases heat. (2) The reaction is exothermic because it absorbs heat. (3) The reaction is endothermic because it releases heat. (4) The reaction is endothermic because it absorbs heat.
1) Start with a balanced chemical equation. 2) Then determine the chemical amount of SO2 from the equation = 2 mol 3) Then use 4) Then report the enthalpy change by writing it next to the balanced equation. (this is an exact #, don't use for sig digs) to determine the enthalpy change for the whole reaction.
(a) Write a balanced thermochem-ical equation for this reaction. (b) Draw an enthalpy diagram for the reaction. 5.40 The decomposition of slaked lime, Ca(OH) 2 (s), into lime, CaO(s), and H 2 O(g) at constant pressure requires the addition of 109 kJ of heat per mole of Ca(OH) 2. (a) Write a balanced thermochemical equation for the reaction.
on the board. Discuss demonstration, write the chemical reaction on the board. (Write Equation Here) Lead a discussion on some common exothermic and endothermic reactions. Elicit examples from students and provide additional examples. Examples include: Mixture of cement and water (exothermic) Melting and freezing of ice (endothermic and exothermic)
This is a HUGE question to answer completely! One answer is 'because they result in a negative change in free energy, delta-G.' This may be as a result of the reaction being exothermic, so the products are more stable than the reactants, or could be a result of an increase in entropy (products more disordered than the reactants), or both of these.
9. Draw an enthalpy diagram for a general exothermic reaction; label axis, reactants, products, and ∆H with its sign. 10. Draw an enthalpy diagram for a general endothermic reaction; label axis, reactants, products, and ∆H with its sign. 11. Write a balanced equation and draw an approximate enthalpy diagram for each of
Write A Balanced Equation For The Formation Of C4h10o Butyl alcohol ... Br, C, O, N, F.Write a balanced equation and write a balanced equation for the formation of c4h10o draw an enthalpy diagram for select if exothermic or endothermic.6% in the urine Write a balanced equation for the combustion of 1 mole of {eq}C_4H_{10}O(l) ...
(a) Write a balanced equation for the complete combustion of propane gas. (b) Calculate the volume of air at 25 °C and 1.00 atmosphere that is needed to completely combust 25.0 grams of propane. Assume that air is 21.0 percent O 2 by volume.
Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): combustion of one mole of methane. vaporization of liquid alcohol. freezing of liquid water. formation of 1 mole of potassium chloride from its elements (heat is released) Learn this topic by watching Energy Diagram Concept Videos.











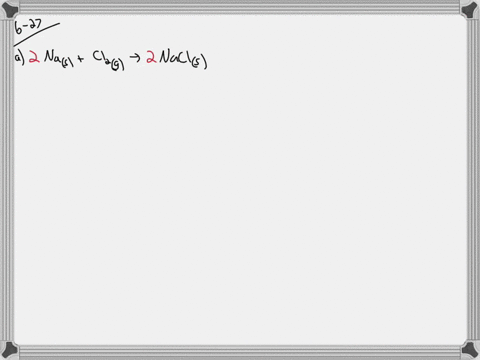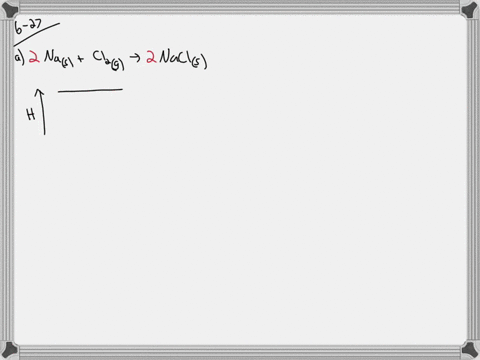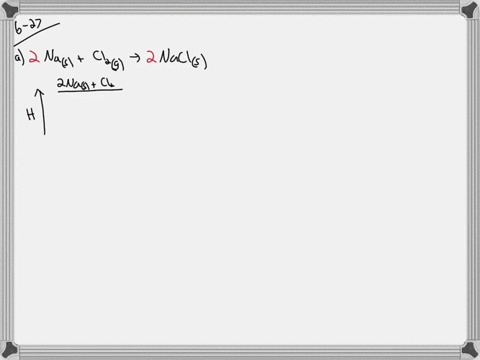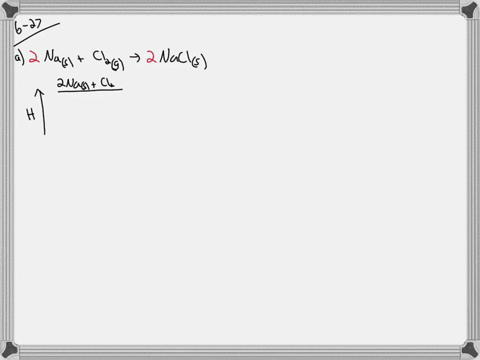





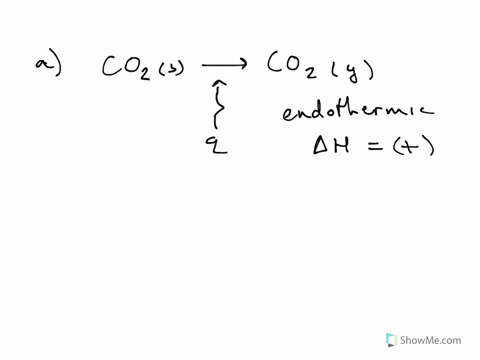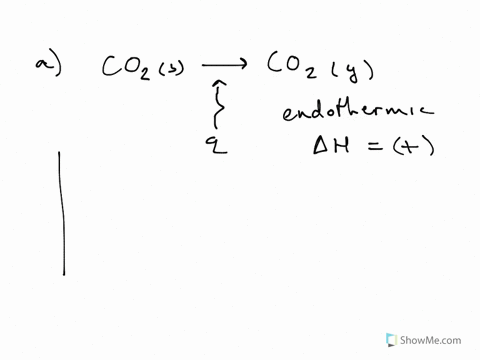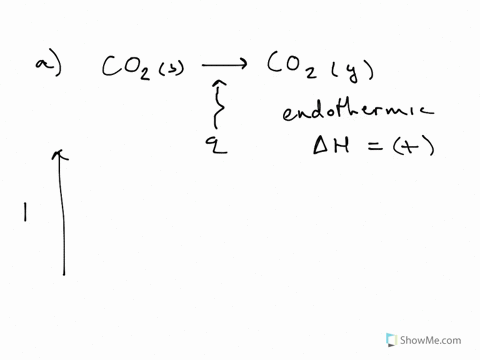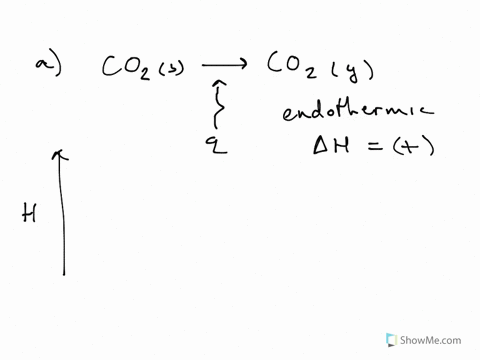
0 Response to "35 write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic):"
Post a Comment