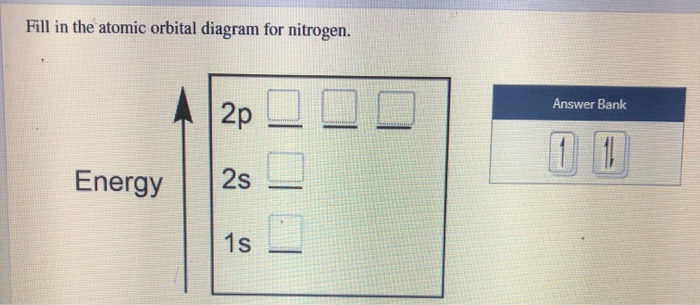
36 atomic orbital diagram nitrogen
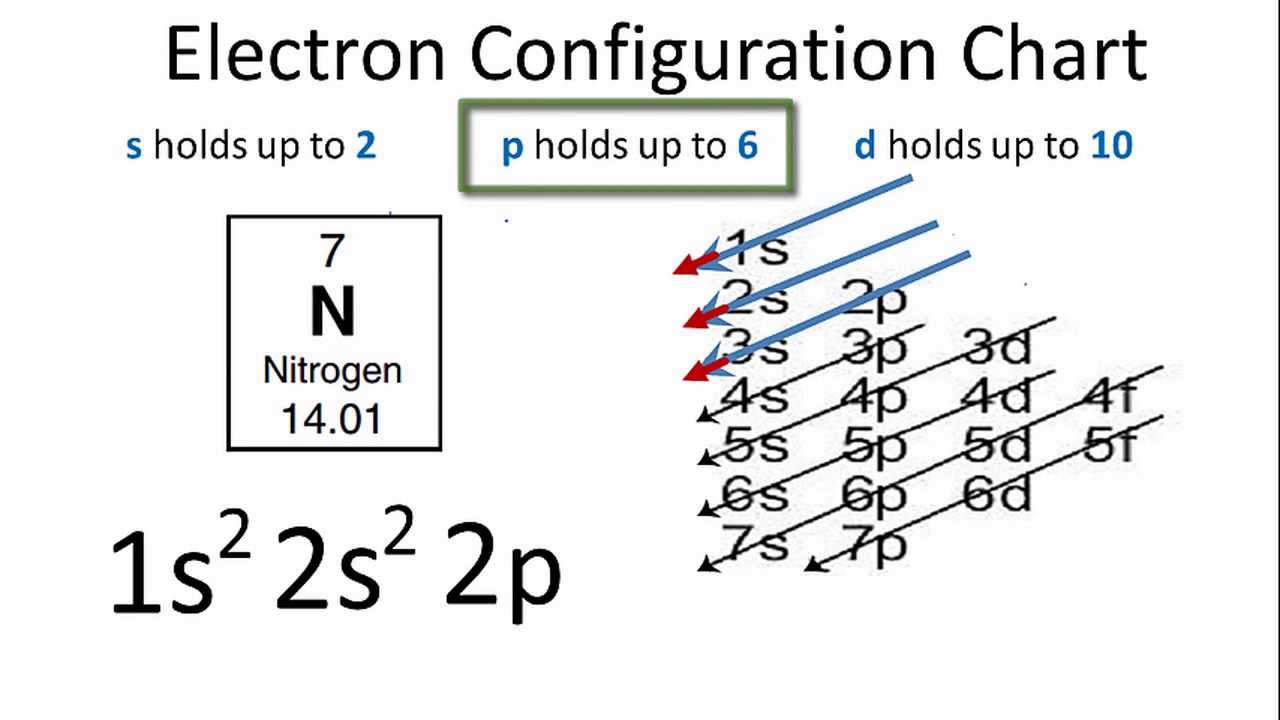
Nitrogen electron configuration is 1s2 2s2 2p3. The two electrons of nitrogen will be in the first orbit and other five electrons will be in the second ...Jul 5, 2021 · Uploaded by Wayne Breslyn
Atomic structure atoms have a nucleus that contains protons and neutrons electrons are. Lets take a look at how to draw bohr diagrams. Bohr model of Fluorine F 2 7. Electron 2P number of protons 2P 1P orbital shells 2N number of neutrons 2N Element symbol He. How to draw Bohr Diagrams a step by step tutorial. Bohr model of Carbon C 2 4.
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation.A material containing unstable nuclei is considered radioactive.Three of the most common types of decay are alpha decay (𝛼-decay), beta decay (𝛽-decay), and gamma decay (𝛾-decay), all of which ...
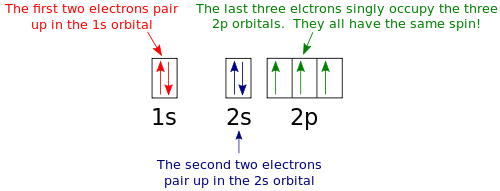
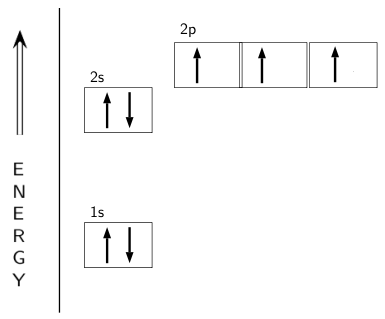
Atomic orbital diagram nitrogen
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ...
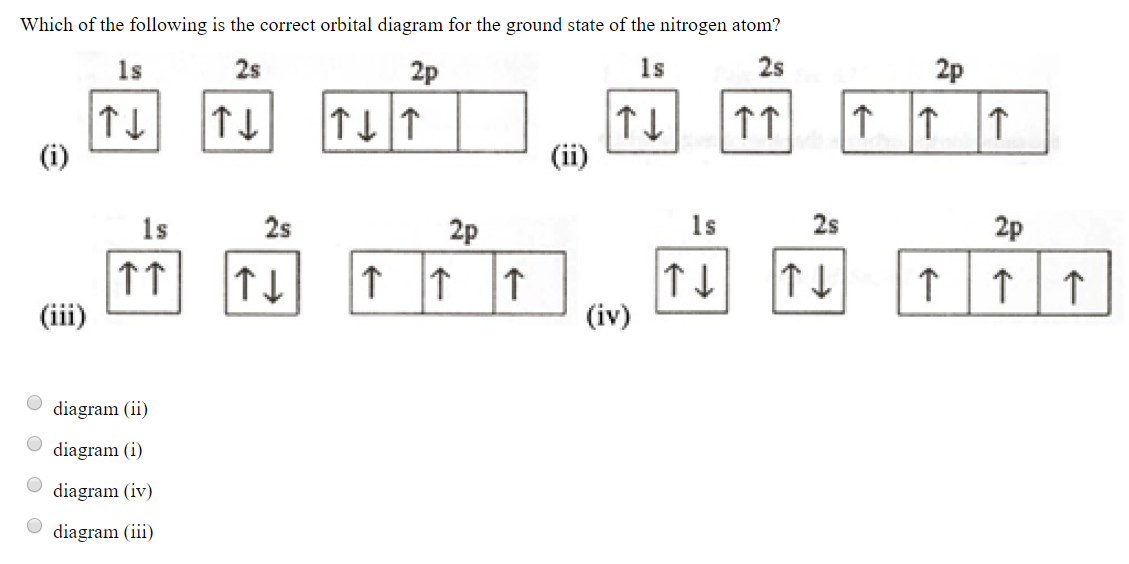
Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals ...1 answer · Top answer: Nitrogen From the periodic table, we can find Nitrogen which has an atomic number of 7. According to the Aufbau priniciple we have to completely...
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
Atomic orbital diagram nitrogen.
Write the electron configuration of NO molecule in the ground electronic state based on this energy diagram. 6o" Sx 20" 21 Y atom orbitals lo NO molecular ...1 answer · 0 votes: Nitrogen rightarrow 7 rightarrow 1 s^2 2 s^2 2p^3 orbital diagram (2) Ni rightarrow 28 rightarrow 1 s^2 2s^2 2p^6 3s^2 3p^6 3d^8 4s^2 orbital diagram:
We study the quantum transport properties of graphene nanoribbons (GNRs) with a different edge doping strategy using density functional theory combined with nonequilibrium Green’s function transport simulations. We show that boron and nitrogen edge doping on the electrodes region can substantially modify the electronic band structures and transport properties of the system. Remarkably ...
The isotopes of cobalt range in atomic weight from 50 u (50Co) to 73 u (73Co). The primary decay mode for isotopes with atomic mass unit values less than that of the most abundant stable isotope, 59Co, is electron capture and the primary mode of decay in isotopes with atomic mass greater than 59 atomic mass units is beta decay.
Oxygen Lewis Dot Structure is surrounded by four dots and two sticks or lines, affecting other 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, providing it an octet and preparing it cell. The two letter O's in the O2 Lewis structure exemplify the nuclei (centers) of the oxygen atoms. Draw the electron dot structure for an oxygen molecule. Oxygen lewis ...
Jan 21, 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...






















0 Response to "36 atomic orbital diagram nitrogen"
Post a Comment