36 lewis dot diagram for sulfur
Lewis Dot Structure For Lithium And Sulfur, Solfuro di litio Wikipedia, Multimedia: Represent Bonding with Lewis Dot Diagrams, Chemistry 101 : Chap 8 PowerPoint Presentation, 31 Lewis Dot Diagram Worksheet Pdf Free Wiring Diagram
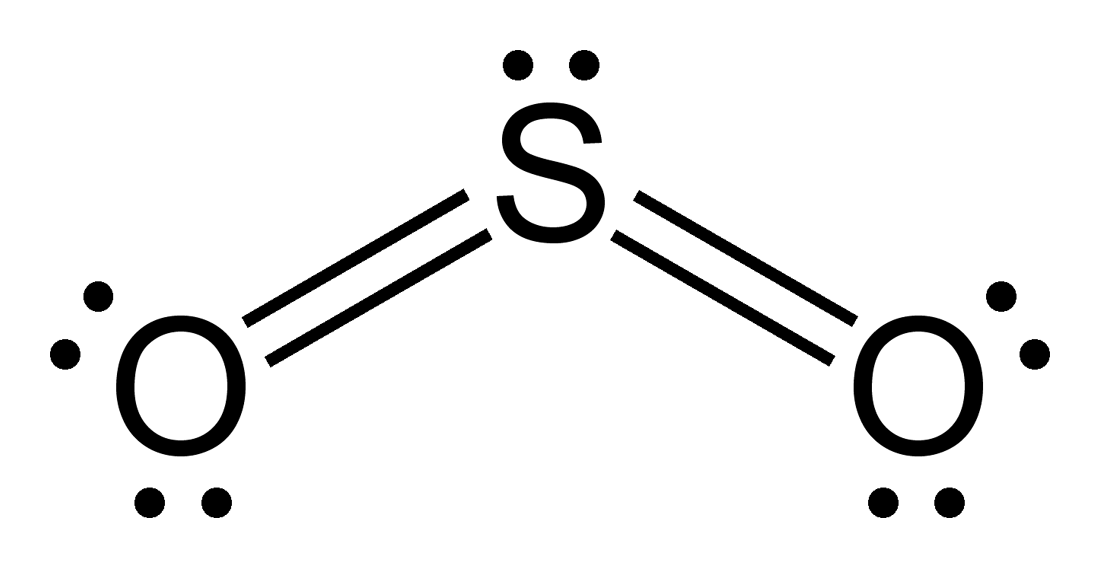
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
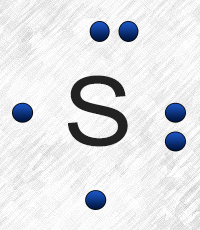
Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons. When you draw the Lewis structure for Sulfur you'll put six "dots" or valance electrons around the element symbol (S). Click to see full answer Furthermore, what is the Lewis dot structure for sulfur?

Lewis dot diagram for sulfur
Lewis dot structure for cas. October 22, 2021 thanh. 2. Draw the Lewis structures for the formation of CaS from the calcium and sulfur atoms (3 pts) 2.
Lewis Dot Structure of SF6 The central atom here is Sulfur as it is less electronegative than Fluorine. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability, which is easier to attain. As there are 6 atoms of Fluorine, there will be a formation of 6 bonds between Sulfur and Fluorine.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
Lewis dot diagram for sulfur.
Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
After that I draw the Lewis dot structure for Sulfur (S). Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons. When you draw the Lewis structure for Sulfur you'll put six 'dots' or valance electrons around the element symbol (S).
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the . Here are the steps I follow when drawing a Lewis structure.
Sulfur And Chlorine Lewis Dot Diagram, Show The Orbital Filling Diagram For Br Bromine Wiring, Lewis Dot Diagram For Potassium, How the ionic bond forms in Lithium Sulfide (Li2S) YouTube, Valence Shell Electron Pair Repulsion
Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry. SF4 has ...
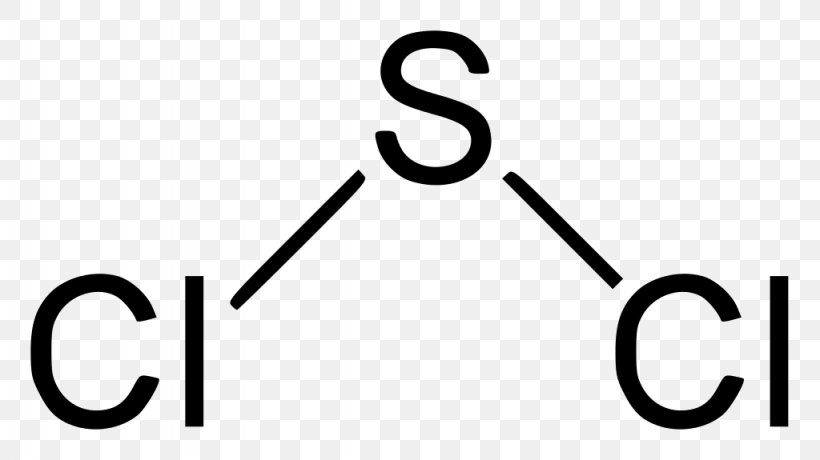
SBr2 Lewis structure is made up of two atoms, sulfur, and bromine, the sulfur is in the central position and bromine atoms are in the surrounding position. The lewis structure of SBr2 contains 16 nonbonding electrons and 4 bonding electrons. The lewis structure of SBr2 is similar to the SCl2 and it is very easy to draw. Here let's see how to ...
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Answer: The formula of Disulfur dioxide - Wikipedia is like this: Lewis structures are not a good model for this molecule: Oxygen and sulfur have 6 valence shell electrons. In total 24 electrons to be drawn ( or 12 pairs. ) 5 pairs are already taken for bonding as displayed above. So 7 pairs l...
SOCl2 lewis structure contains a sulfur atom in central position whereas the two chlorine and one oxygen atom spread evenly around it. There is one double bond and two single bonds present in the lewis structure of SOCl2. The lewis dot structure of SOCl2 disobeys the octet rule for attaining stability, let's see how to draw this in a simple way.
Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol ...
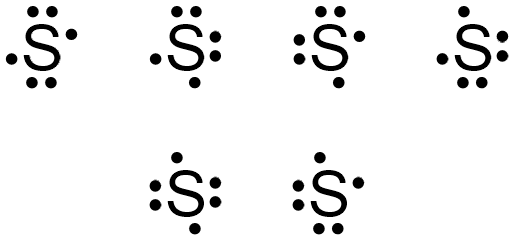
Sulfur (S) has six valence electrons. A Lewis dot structure of around 'S' would have two dots on two sides, and one single on each of the remaining. It would look be drawn as: .. .S : .
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
Lewis dot structure will have 4 paired dots around Sulfur atom.For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure. Lewis dot structures for Polyatomic ions and molecules : However for molecules and polyatomic ions we need to consider many more factors before drawing a correct Lewis dot structure.
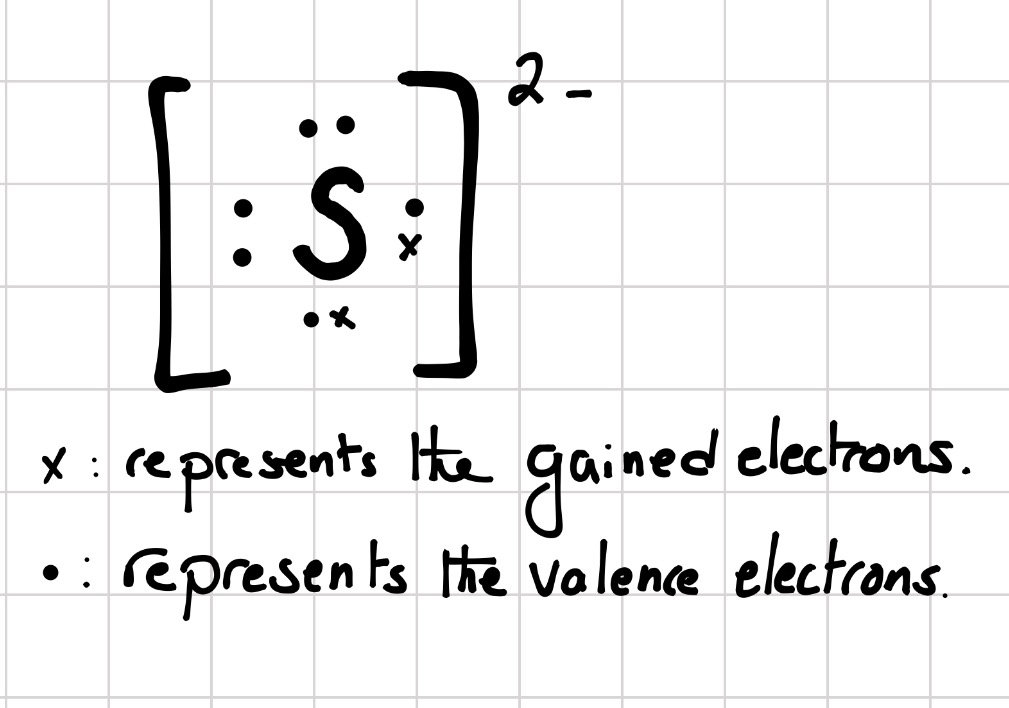
Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
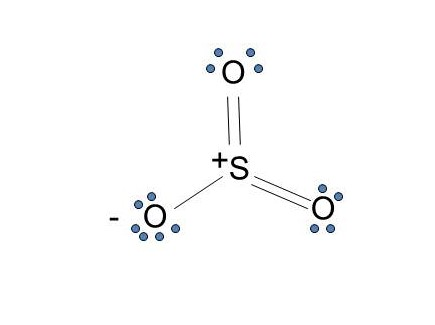
Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
A step-by-step explanation of how to draw the SI2 Lewis Dot Structure.For the SI2 structure use the periodic table to find the total number of valence electr...
One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO 2 molecule.
A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Lewis structure of SO3. The sulfur trioxide is a tetra atom chemical molecule whereby both the sulfur and also three oxygen molecules bond with an equal variety of valence electrons. The chart is attracted showing dots the valence electrons roughly the prize of both sulfur and also oxygen atoms through lines predicting link formation.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Lewis Dot Diagram For Sulfur - Atkinsjewelry from hi-static.z-dn.net Metallic elements, to the left of the staircase dividing line, tend to loose one or more electrons and form ions. In total 24 electrons to be drawn ( or 12 pairs. The total number of valence electrons for s is 6 (sulfur is also in the 6th column of the periodic table).




















0 Response to "36 lewis dot diagram for sulfur"
Post a Comment