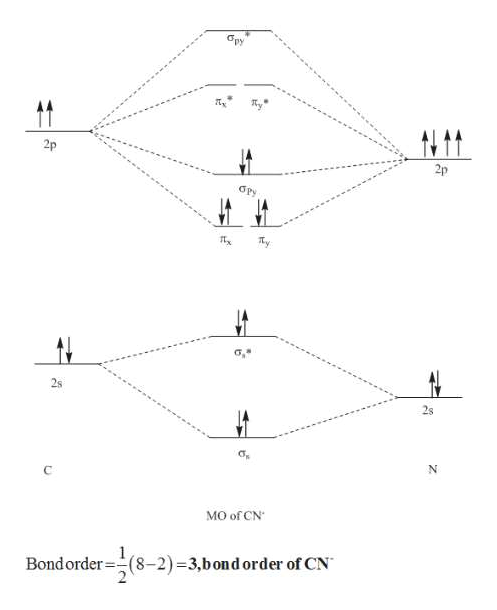
36 molecular orbital diagram for cn-
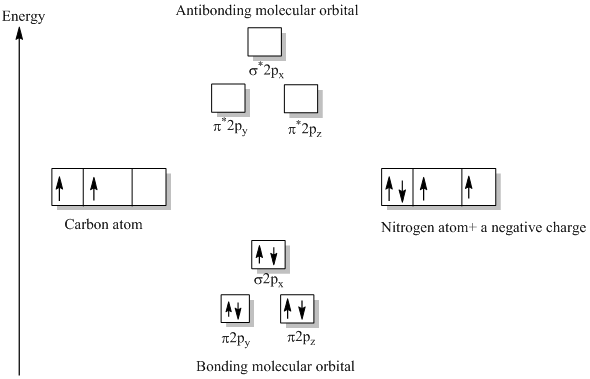
(b) Given the atomic energy levels for the carbon and nitrogen atoms below, draw a molecular orbital diagram for CN- anion labeling all the bonding and antibonding molecular orbitals appropriately. Fill in the electrons in the appropriate orbitals. 2p __ __ __ 2s __ 1s __ C __ __ __ 2p __ 2s __ 1s. N
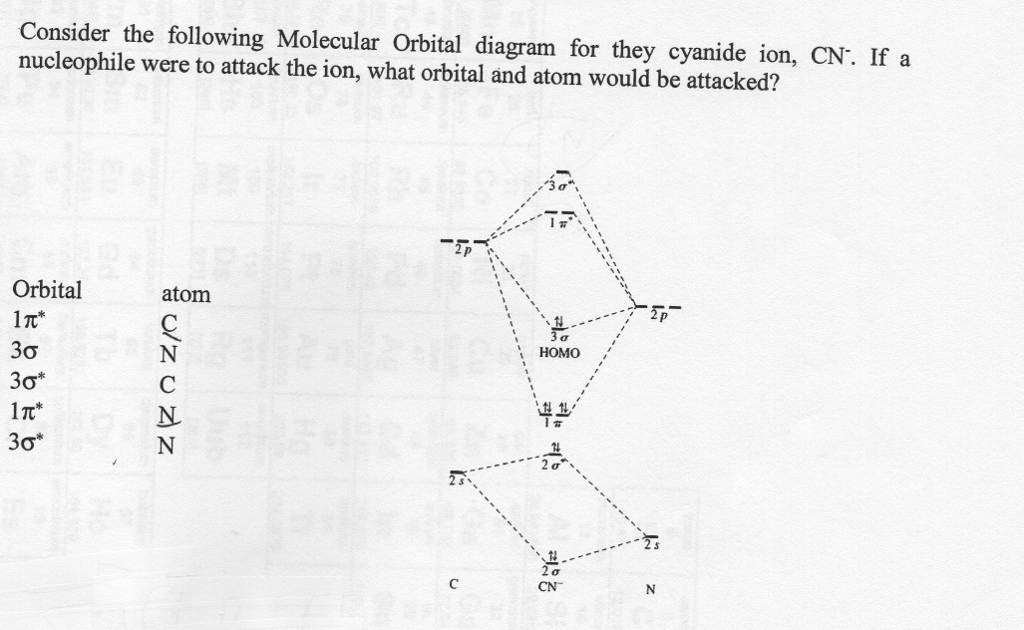
The MO diagram of CN is similar to N2. Based on the molecular orbital energy-level diagram of CN, which of the following statements are correct? i. The CN bond order is 2. ii. CN is diamagnetic. iii. The bond enthalpy in CN- ion is higher than CN. iv. CN has longer bond length than CN-answer choices: a. ii, iv b. iii, iv c. i, iii d. i, ii e. i, iv
We're being asked to complete the molecular orbital diagram of CN- and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate ...17 Sept 2020

Molecular orbital diagram for cn-
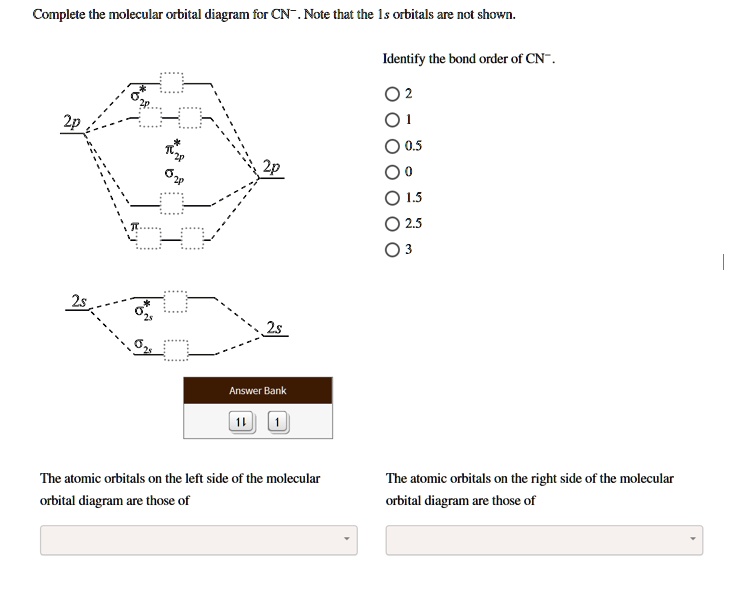
Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital and the other is called an anti-bonding molecular ...1 answer · Top answer: Concepts and reason • The molecular orbital theory explains the bonding in terms of the combination and organization of atomic orbitals of an atom which ...
Molecular orbital diagram for cn-. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular orbital diagram for cn-.
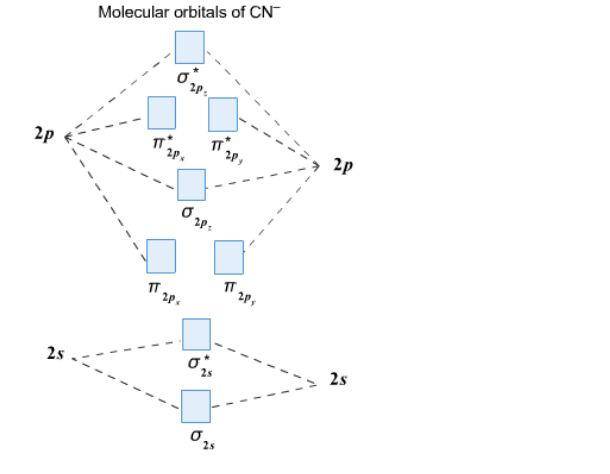
Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the Molecular orbital diagram of CN-we can ... A) The total number of molecular orbital s for med doesn't always equal the number of atomic orbital s in the set. B) A bond order of 0 represents a stable chemical bond.
The molecular diagram you would use is the Z<8 one because carbon's atomic number is 6, however when filling in the valence electrons you would use 10 because it is two carbons ( C2) (4+4) with a negative 2 charge so (+2 valence electrons). The bond order of this would be calculated using the middle pi and sigma bonds.
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...
In the molecular orbital diagram for . O_(2)^(+) ion the highest occupied orbital is . 127322982. 200+ 5.5 k+. 02:55. In the molecular orbital diagram for `O_(2)^(+)` ion the highest occupied orbital is . Total number of molecular orbitals containing electrons present in . ... (CN)_6]^(3-)sp^(3)d^(2)
The molecular orbital theory is highly dependent on the geometry of the complex and can successfully be used for describing octahedral complexes, tetrahedral and square-planar complexes. The main features of molecular orbital theory for metal complexes are as follows: Representative d-orbital splitting diagram s for square planar complexes ...
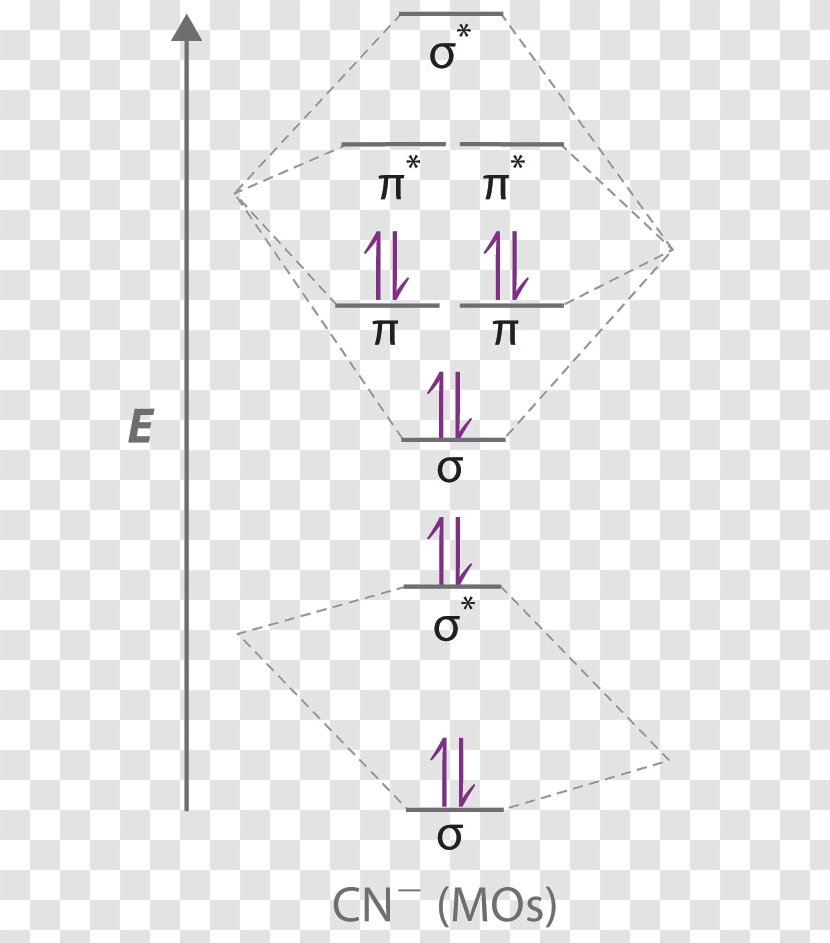
Nov 21, 2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The ...
4. a. Which of these complexes would be best explained by a pi-donor molecular orbital diagram? b. Using a molecular orbital energy-level diagram, show the interactions that result in ?o for a pi-donor octahedral metal orbital. (You can start with a sigmaonly diagram to the left). c.
38 hcn molecular orbital diagram; 38 drag the labels onto the diagram to identify th... 37 mo diagram cn-37 entity relationship diagram powerpoint; 38 2003 buick lesabre serpentine belt diagram; 37 remote start wire diagram; 40 lennox furnace parts diagram; 40 smart car parts diagram; 38 drag the labels onto the diagram to identify th...
Arrange the following molecular species in increasing order of stability. Answer: N 2 2-< N 2-= N 2+ < N 2. Question 48. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic? Answer: O 2 molecule contains one unpaired electron in each of one π2p x and π2p y orbitals. Question 49. Define antibonding molecular ...
Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. 2 linear.
orbital from the σ-bonding. The molecular orbital of t2g* are greater in energy than nonbonding sets of t1g, t2u and t1u. On the other hand, the bonding molecular orbitals of t2g are lower in energy than that of nonbonding SALCs of the ligands. Therefore, when 36 electrons (12×2 from π-bonding and 6×2 from σ-bonding) from ligands are ...
Dec 16, 2021 · The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of ...
CN- lewis structure, molecular orbital diagram, bond order … The bond order of … O2, B2, etc. ⇒ A hetero-nuclear diatomic molecular orbital in which different atoms combine together. example- CN, HF, NO, etc. Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms.
Now, let us study the steps involved in drawing the Lewis Structure of methylamine (CH3NH2): Step 1: Find the total number of valence electrons methylamine already has: It is 14 for one methylamine (CH3NH2) molecule as 4 are coming from the carbon, 1 from each hydrogen atom, and 5 from the nitrogen atom. Step 2: Find for how many more valence ...
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...
Answer: I don't know as if you have an idea about coordinate bond or not but I will try to make it easy. You may aware of two type of typical bonds 1. Ionic bond - Complete transfer of 'a electron or more' to most electronegative element. Number of electrons = number of bond formed. Mostly seen...
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbital s method in particular. C would this ion exist.
Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3.
The sp orbitals of both C and N combine to form the sigma bond in C⦀ N. Molecular Orbital Diagram Molecular Orbital Theory is slightly different from VBT and orbital hybridization. Here, AOs from different atoms inside the molecule can come together to form molecular orbitals or MOs. Therefore, valence electrons are shared inside the molecule.
Molecular Orbital Diagram s, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solut ion. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Molecular Weight: in chemistry of a substance (sometimes called the molecular weight of a substance) is the mass of a molecule of that substance, relative to a unit of atomic mass (u which equals 1/12 of the mass of an n-carbon-12 atom) (simply: molecular mass is the sum of the weights atoms in a molecule). Molecular mass can be calculated as ...
The total energy of the molecule must be stabilised. This means that very occasionally an occupied MO can move up in energy as long as the occupied MOs move ...
In addition to interacting with s and p orbitals of ligands such as NH,, CN, etc., d orbitals can also interact with other d-orbitals, resulting in the formation of metal-metal bonds. Thus, for a simple metal dimer (i.e., ignoring any other ligands), we can envision the following interactions: M1M2 ? bond bond 8 bond where the o bond represents ...
The molecular orbital diagram consists of two types of bond. They are sigma bond (σ) \left( \sigma \right) (σ) and pi bond (π) \left( {\rm{\pi }} \right) (π). The electrons first fill the lower energy level 2s and then moves to the higher energy level 2p. Also, the electrons first fill the bonding molecular orbital and then moves to anti ...
Molecular orbital (MO) theory explains the construction of molecular orbital diagram on the basis of following main points . 1.Formation of MOs: Atomic orbitals(AOs) linearly combine with each other to form equal number of molecular orbitals (MOs). 2.Energy of MOs: Half of the molecular orbitals (MOs) having energy lower than the atomic orbitals are called…
Molecular orbital theory (MO theory) can be a very challenging topic. Students come into the classroom already knowing about the octet rule, Hund's rule, and the Pauli exclusion principle. However, the lecture component is critical for bridging the gap between their knowledge of general chemistry and organic chemistry. As such, I start my lecture on…

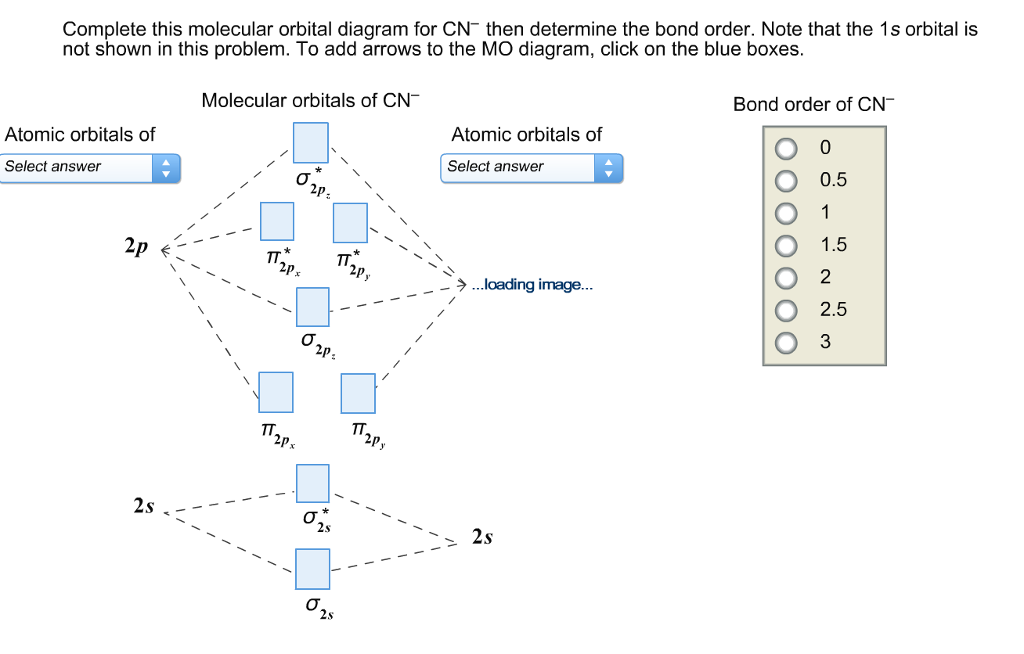
Complete this molecular orbital diagram for cn– then determine the bond order. note that the 1s orbital is not shown in this problem.
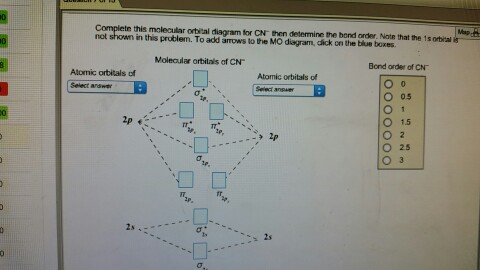
Complete this molecular orbital diagram for CN then determine the bond order. Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes. Bond order of CN O 0 O 0.5 Molecular orbitals of CN Atomic...
Complete this molecular orbital diagram for CN then determine the bond order. Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes. Bond order of CN O 0 O 0.5 Molecular orbitals of CN Atomic...
Molecular orbital diagram practice worksheet. For the following elements. the four orbitals as bonding, non-bonding or antibonding. 1 and inserting your answers in the answer blanks. Nitrogen is the seventh element with a total of 7 electrons. 30 M solution of NaCl (58. 69 m 50. The HR Diagram. Molecular Orbital Worksheet 1. 54 m C) 17.
MPA 44, 2nd floor, Rangbari Main Road, Mahaveer Nagar II, Kota (Raj.) - 324005
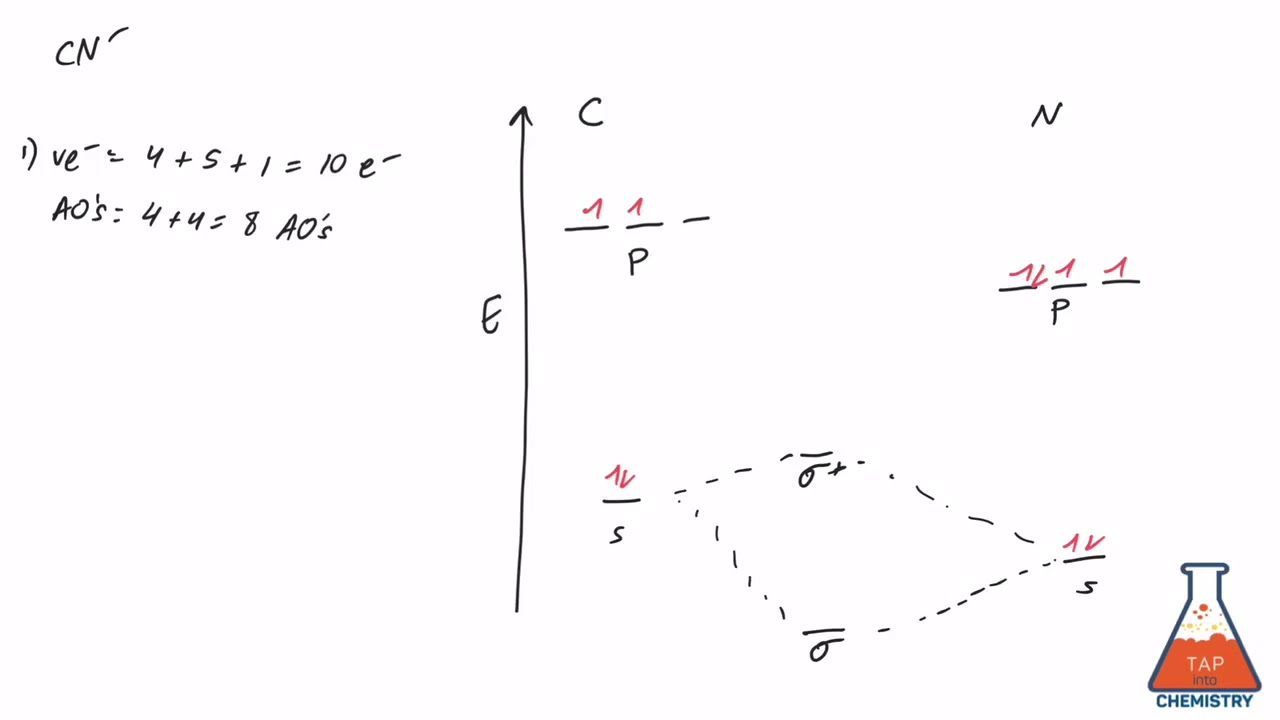
Answer: Let's consider the valence electrons for each atom: C: 2s22p2 N: 2s2p3 Overlapping of s orbital will not contribute to the formation of bonds since they are full. Let's consider the remaining p electrons: 2 + 3 + 1 = 6. 1 electron is added for the negative charge -. Overlapping of p ...
Question: Use Molecular Orbital Theory To Determine Whether He2 Or He2+ Is More Stable. These properties can be explained by the molecular orbital diagram of BN". The bond order of two suggests that the oxygen molecule is stable. Correct option (a) O-2. Diamagnetic Metals + properties give you a broad overview of these metals from multiple angels.
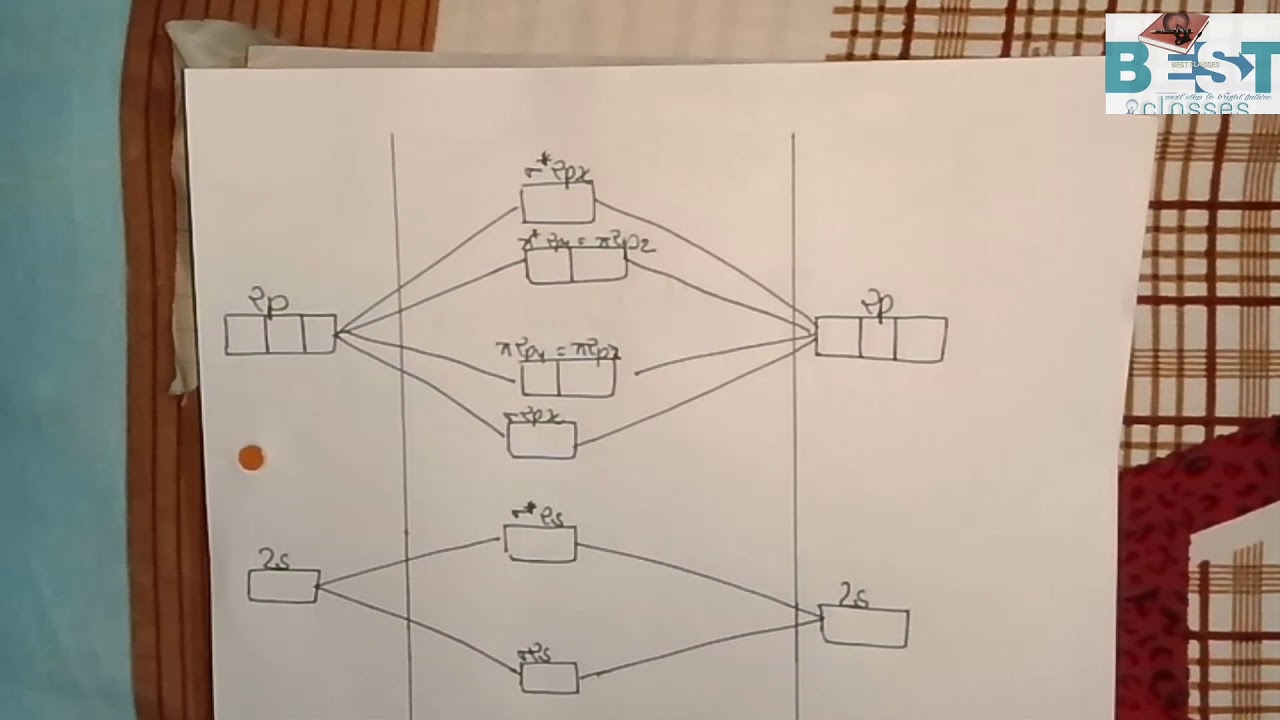
6 answers[math]\text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2}[/math] Here is the molecular orbital diagram of CN-: There ...
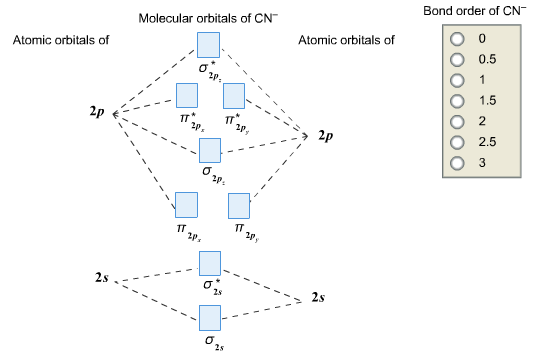
Complete this molecular orbital diagram for cn– then determine the bond order. note that the 1s orbital is not shown in this problem.













0 Response to "36 molecular orbital diagram for cn-"
Post a Comment