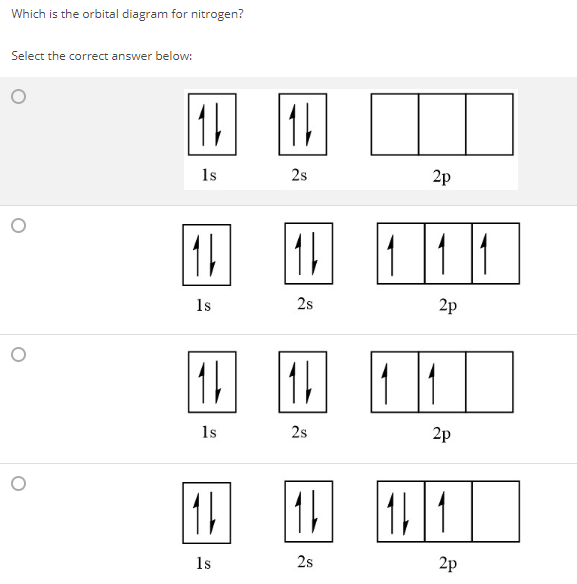
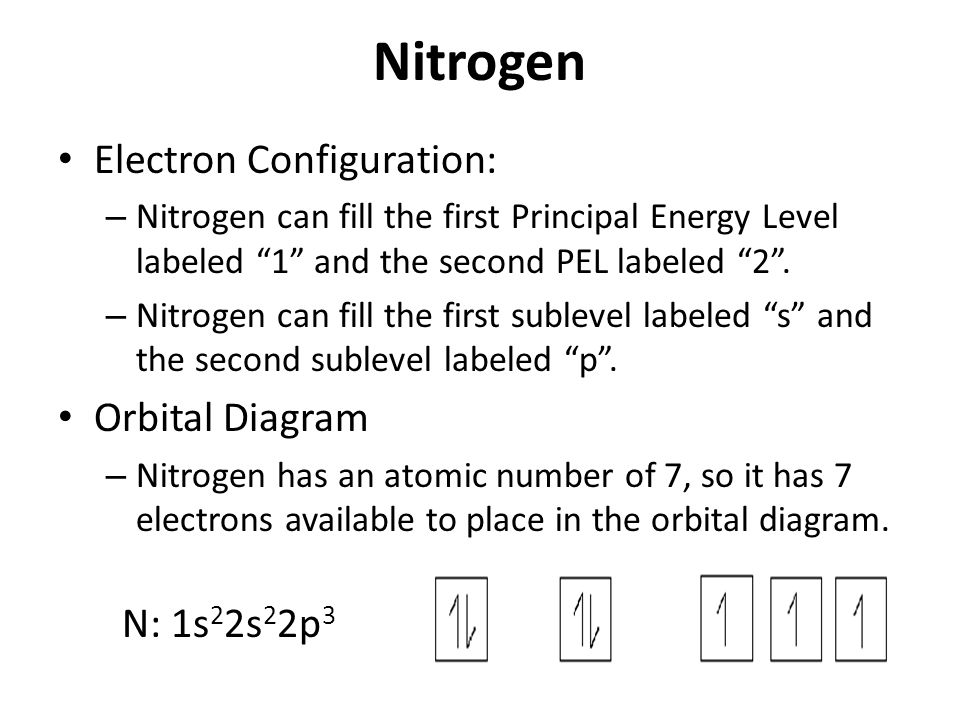
36 orbital diagram for nitrogen
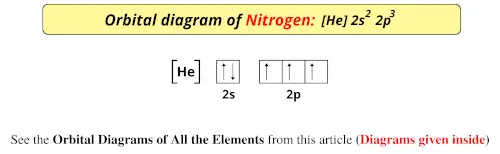
The molecular orbital energy level diagram of He 2 (hypothetical) is given in Fig. Here, N b = 2 and N a = 2. Bond order = N b -N a / 2 = 2-2 / 2 = 0. As the bond order for He 2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N 2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s 2 2s 2 2p 1x 2p ...
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
The molecular orbital energy level diagram of N 2 is given in fig. The bond order of N 2 can be calculated as follows: Here, N b = 8 and N b = 2 Bond order = 2 N b − N a = 2 8 − 2 = 3. Nature of bond: A bond order of 3 means that a triple bond is present in a molecule of nitrogen.
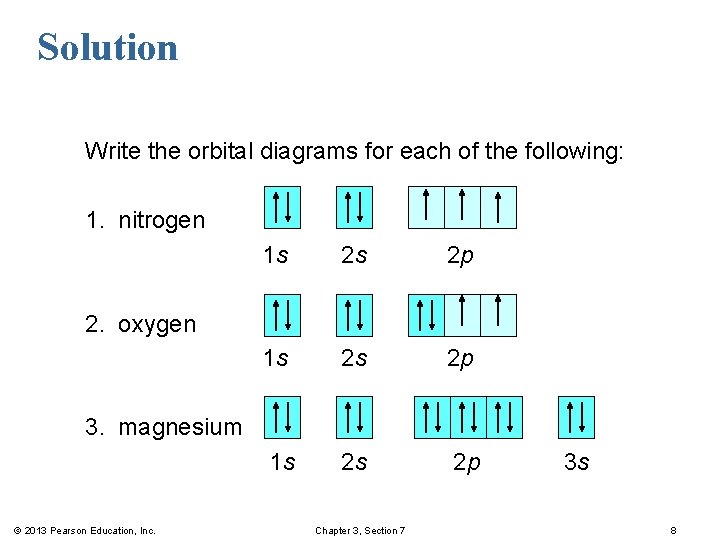
Orbital diagram for nitrogen
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Hydrogen (H) Electron Configuration with Full Orbital Diagram. Hydrogen electron configuration is 1s 1. Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.
Answer: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it: What will be the molecular orbital diagram for nitrite ion? The outcome, i.e. the molecular orbital diagram for Nitrogen dioxide NO₂, should loo...
Orbital diagram for nitrogen.
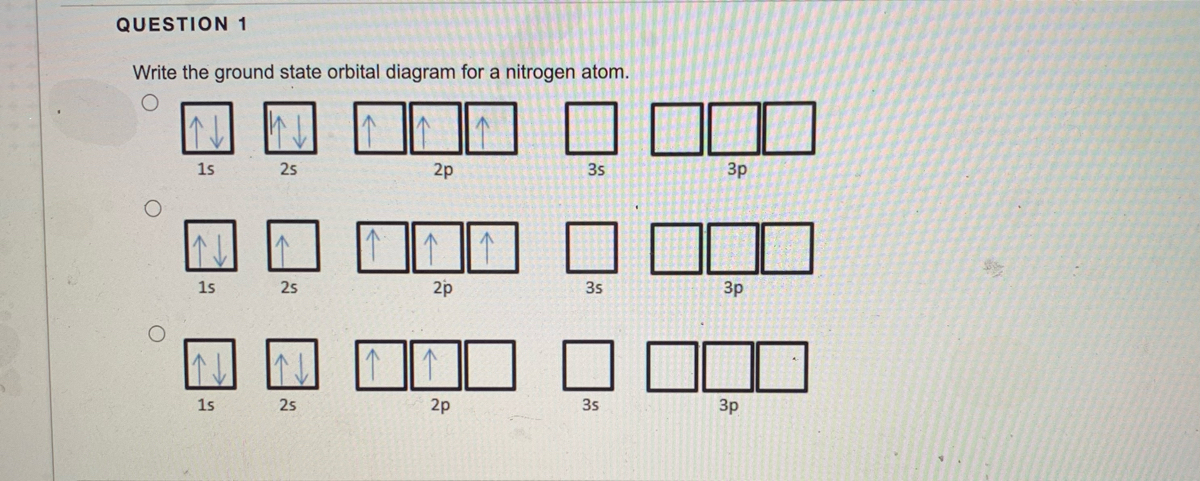
This problem has been solved! What is the orbital diagram for the valence electrons in a ground state atom of nitrogen? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ans :- The orbital dia ….
3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds.
Orbital diagram nitrogen.svg... atom, orbital diagrams, Orbital representation ... Diagram 1 shows the orbitals ..... electrons occupy orbitals ...
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
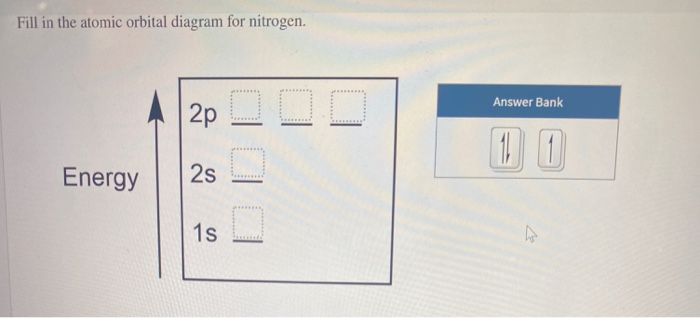
Create the atomic orbital Diagram for Nitrogen. solved create the atomic orbital diagram for nitrogen st answer to create the atomic orbital diagram for nitrogen start by adding the appropriate subshells for example boron is in the create the atomic orbital diagram for nitrogen in some cases we may need to slightly alter the design color or even accessories we want a new thought for it then ...
Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma...
Here is the full molecular orbital diagram for N 2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively.
Molecular orbital diagram of nitrogen gas is shown in the image. Since there are 7 electrons present in one nitrogen atom, so 14 electrons are...
Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...
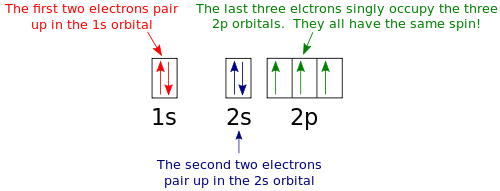
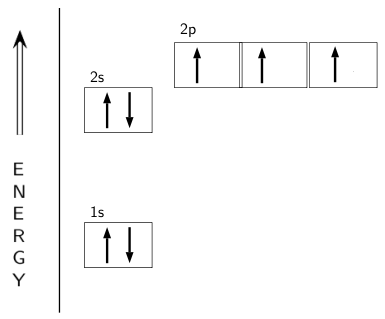
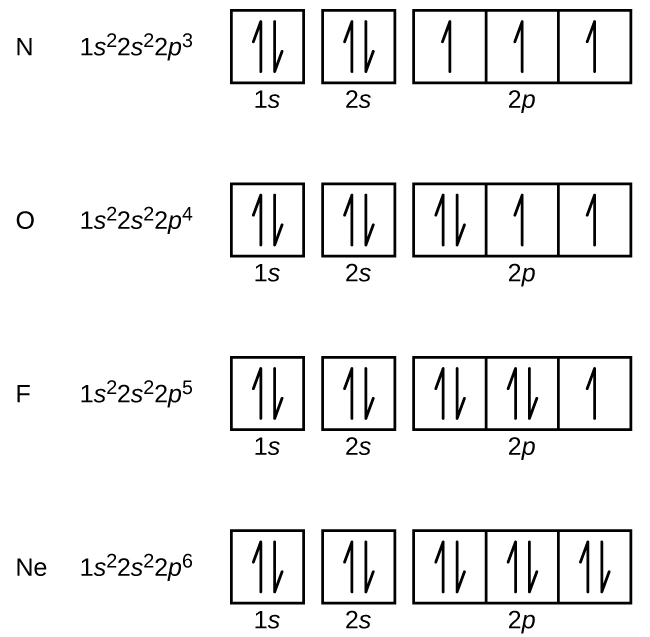
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
Orbital Diagram Of Nitrogen. molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory molecular orbital theory home faculty molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms that is in
Molecular orbitals in Nitrogen. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two ...
To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor.org/
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
09/06/2017 · molecular orbital diagram for nitrogen gas (n2)use aufbau and hund to fill with 10 valence electronsyou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).bond or. For the second period elements the 2s and 2p orbitals are important for mo considerations. 31/03/2021 · 12+ n2 molecular orbital diagram. 07/08/2021 · do you know !!
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom.
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
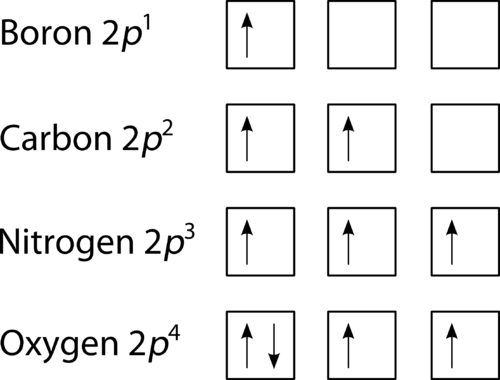
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
The atomic number of nitrogen is 7 and its symbol is 'N'. The standard atomic mass of nitrogen is 14.006. The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article.
What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it.













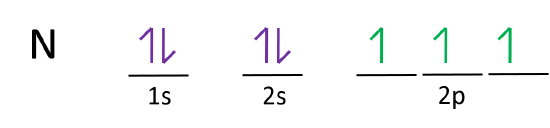



0 Response to "36 orbital diagram for nitrogen"
Post a Comment