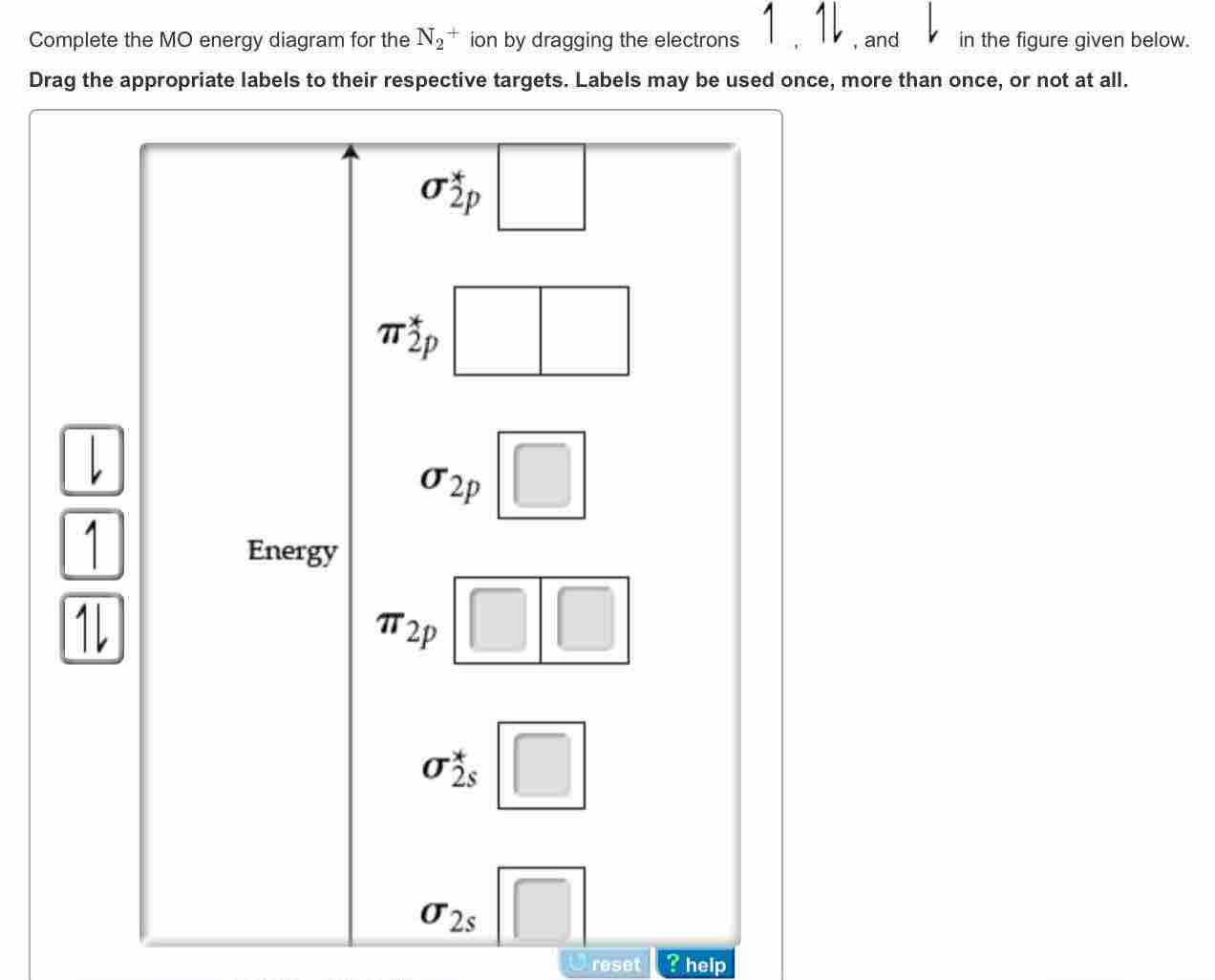
37 molecular orbital diagram for n2+
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Download scientific diagram | Schematic molecular orbital diagram for octahedrally coordinated Fe(II)-chloride complexes. The d → d transition is shown as a light double arrow and possible charge transfer transitions are shown as heavy arrows. This diagram and Fig. 2 were constructed based on...
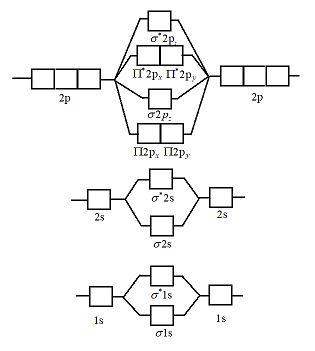
Slide Number 50. Molecular Orbital Approach to Bonding. In molecular orbital (MO) approach - overlap orbitals for the whole molecule -bonding is therefore DELOCALISED. Before moving on to show the energy level diagram for A2 molecules - we need to be clear about the labels for m.o.'s.

Molecular orbital diagram for n2+
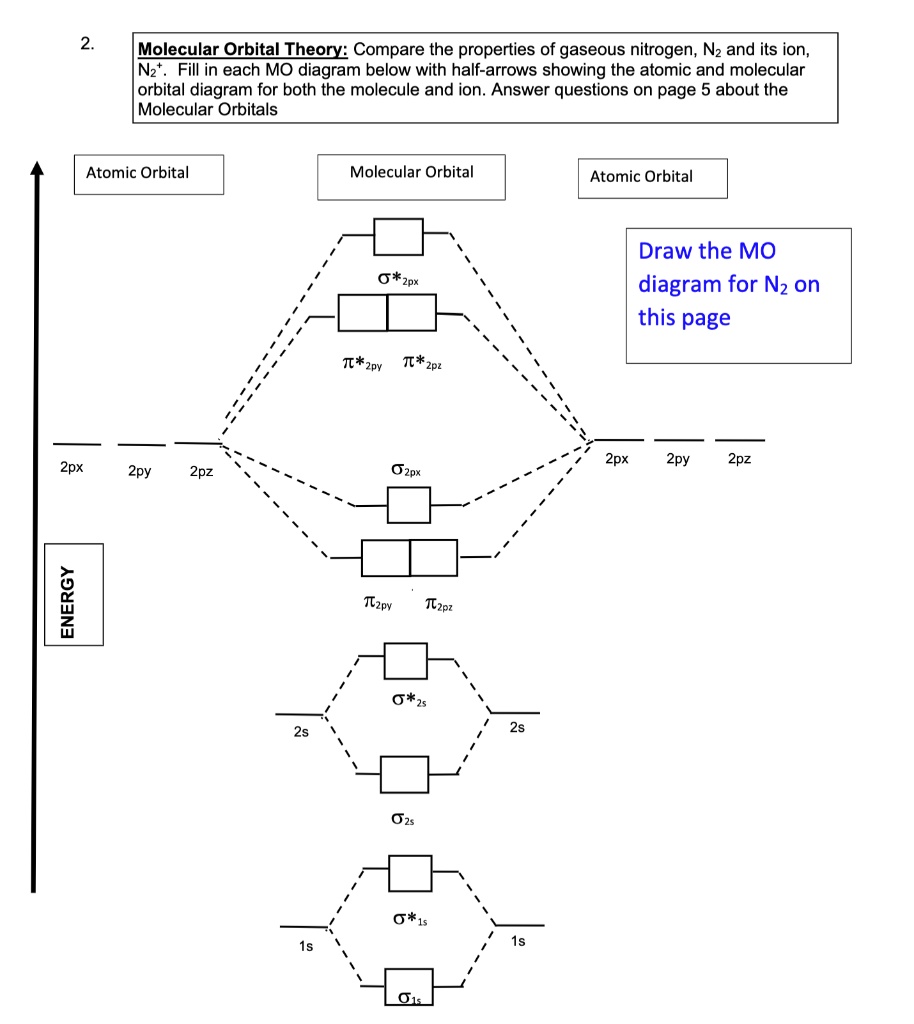
Molecular Orbital Diagram for Nitrogen Gas ( 1 ion) (N2( )). Fill from the bottom up, with 9 valence electrons total. Comparing and Contrasting the Molecular Orbital Diagrams of N2- and N2+. In this video lecture Molecular Orbital Energy Level Diagrams for N2, N2 , N2-, N2 2- C2 and B2 are...
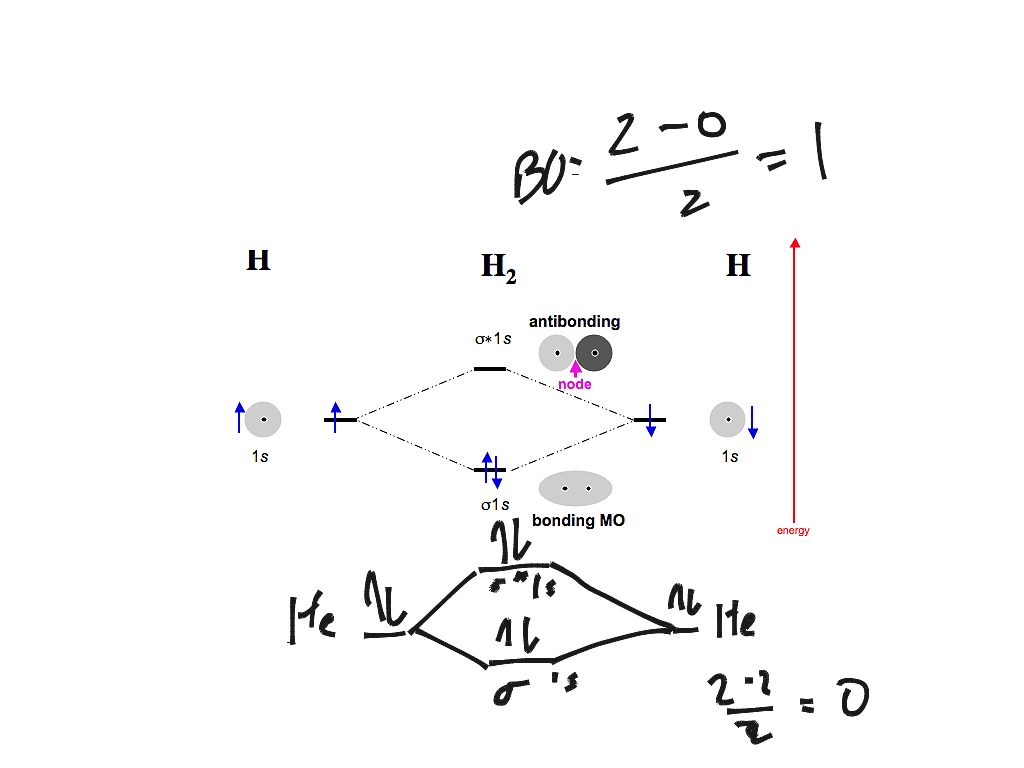
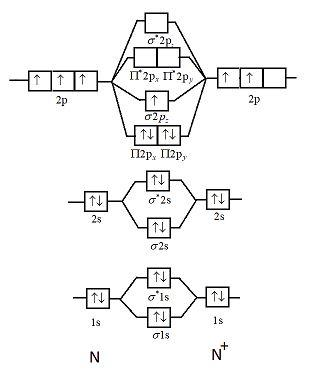
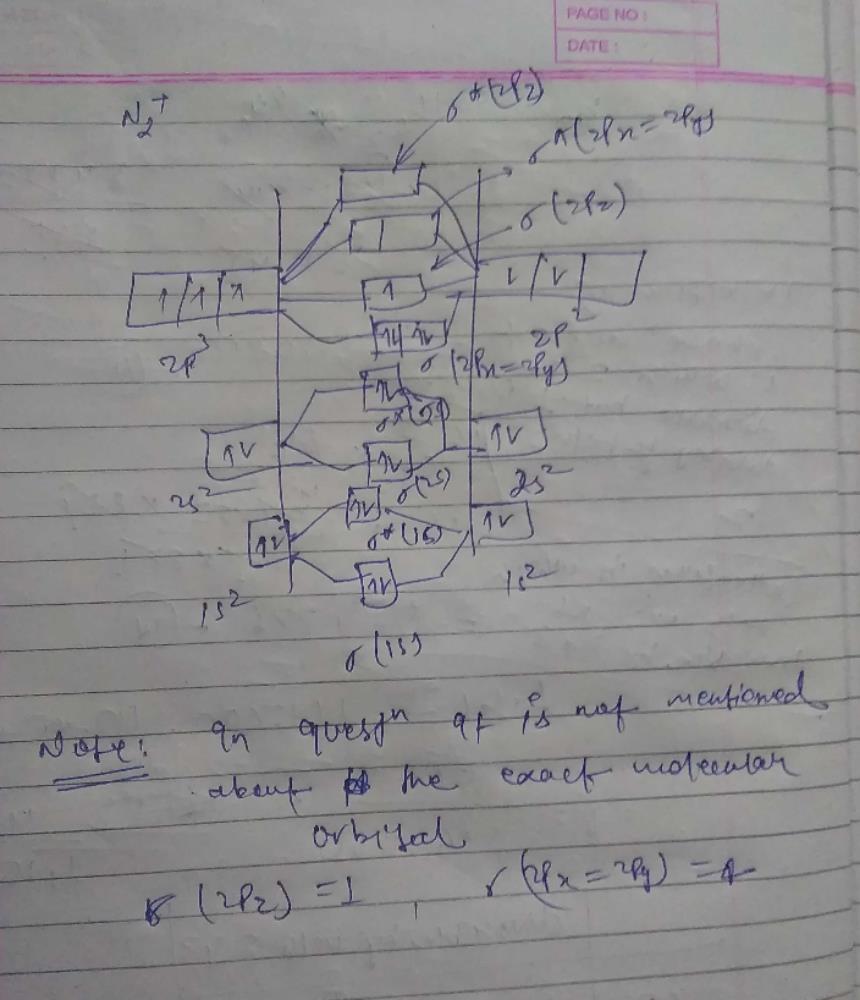
Fig. No. 1 Molecular Orbital Diagram for H2 molecule. Comparison of N2 and N2+ ion N 2 + ion is formed when one electron is removed from N 2 molecule. This electron will be lost from 2p z M.O. Hence the electronic configuration of N 2 + ion will be.
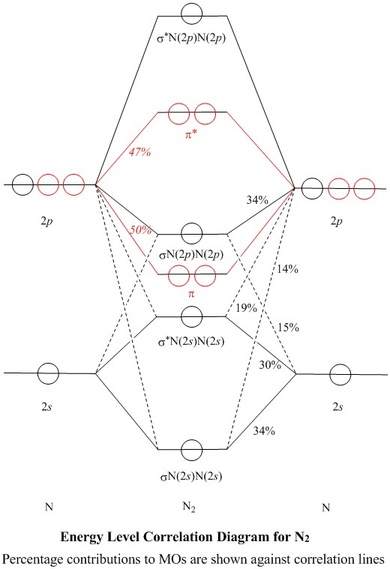
N2 molecular orbital diagram and titled. Interact and form molecular orbitals. 9 8 Second Row Diatomic Molecules Chemistry...
Molecular orbital diagram for n2+.
N2+. Lewis Structure: Molecular Orbital Energy Diagram. Total # of bonding electrons. # of Sigma Bonds. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as...
The molecule is diamagnetic. The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says.
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. ( i have no faqing idea man, how do u determine this again??) For a diatomic molecule, the molecular orbital diagram will contain: the atomic orbitals of each atom the molecular F2+ C2+ H2+ N2 N2+.
The simplest molecule: H2+. Bonding and antibonding orbitals. Simple molecular orbital diagrams. Dihydrogen and its ion H2+. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those...
Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the...
On the left you can see all of the orbitals. 2 e in the sigma 2s bonding orbital lowest energy mo 2 e in the sigma 2s anti bonding orbital...
The mo picture of homonuclear diatomic molecules depends on the amount of sp mixing. On the right the total valence electrons 4 from c 6 fr...
N2 2- Molecular orbital Diagram. molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo diagrams you need to memorize for diatoms.
6. According to molecular orbital theory, which of the following will not be a viable molecule ? 8. Which of the following best describes the diagram below of a molecular orbital ?
Molecular Orbital Diagram For N 2. Molecule Enetsy Level Diagram including each atom's energy levels) Molecular orbital clectron. (a) N atom orbitals and their linear combination to form ... In the formation of N2+ from toppr.com. Molecular Orbital Diagram For N2 - Ekerekizul.
We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+. Draw the molecular orbital energy-level diagram for the system. B Determine the total number of valence electrons in the He22+ ion.
Molecular Orbital Theory & MO Diagrams. Molecular Orbital theory - Chemical Bonding & Molecular Structure. Video | 13:38 min.
Similarly, with Be2+ as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram. Remember, valence electrons are those which do not represent a noble-gas-like-state. the 1s Orbital is full (2 electrons), so the Be2+ configuration is the same as Helium. the 2s orbital in Be is...
N2 molecular orbital energy level diagram also has the following tags. The result is a slight change in the relative energies of the molecu...
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
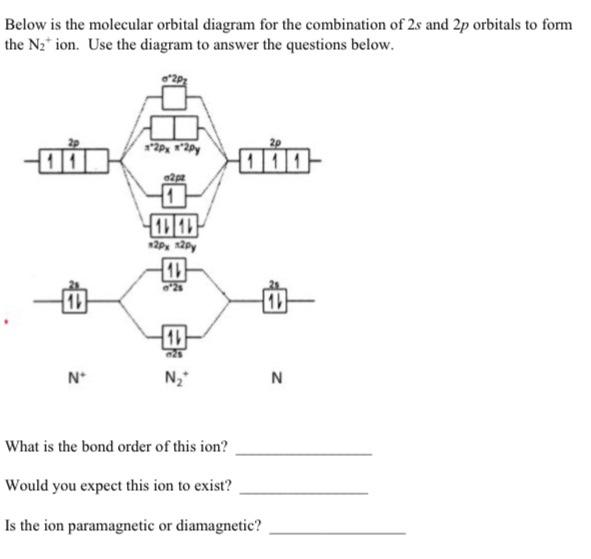
Chemistry questions and answers. Draw molecular orbital diagram of N2+ and what is the formal bond order?
> Molecular orbital theory was developed mainly by. > From elementary molecular orbital theory we can give the electronic conofiguration of the singly positive nitrogen molecular ion N2+ as.
mo-diagram-for-n2-molecular-orbital. Welcome toClipfrom. Interactive video lesson plan for: MO Diagram for N2+ (Molecular Orbital). Activity overview
I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
There are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc. When the electronegativity of one atom is lower than the o...
mmmm.... that is not a molecule or a molecular orbital diagram syko sykes, try again! here's a M.O. diagram of N2+, it should be a little more helpful than my previous post
**Physical Chemistry** **Thermodynamics, Structure, and Change 10th Edition Solutions Peter Atkins, Julio de Paula** **ISBN-13: 9781429290197** Download the Solutions manual for this textbook **Order it via email: markrainsun"@"gmail(.)com** ​ **Table of Contents** **Foundations** A Matter B Energy C Waves **Part 1 Thermodynamics** **1. The properties of gases** Topic 1A The perfect gas Topic 1B The kinetic model Topic 1C Real gases **Impact** …O...
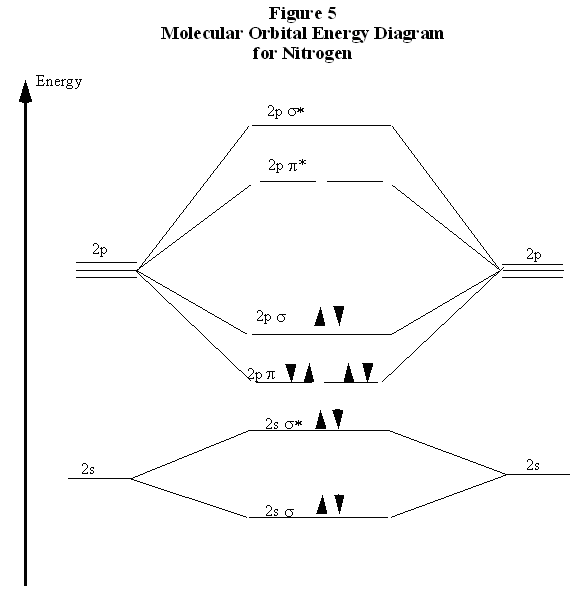
Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂...
At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne. My lectures state that this is due to s and p mixing and my textbook states that it is due to electron repulsion ...
Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)). • Molecular orbital diagram and their electronic configuration for N2, N2+, N2





![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)





.png)










0 Response to "37 molecular orbital diagram for n2+"
Post a Comment