38 diagram of exothermic reaction
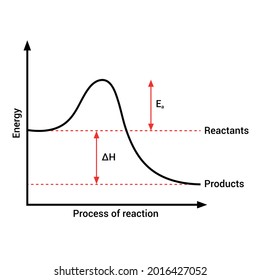
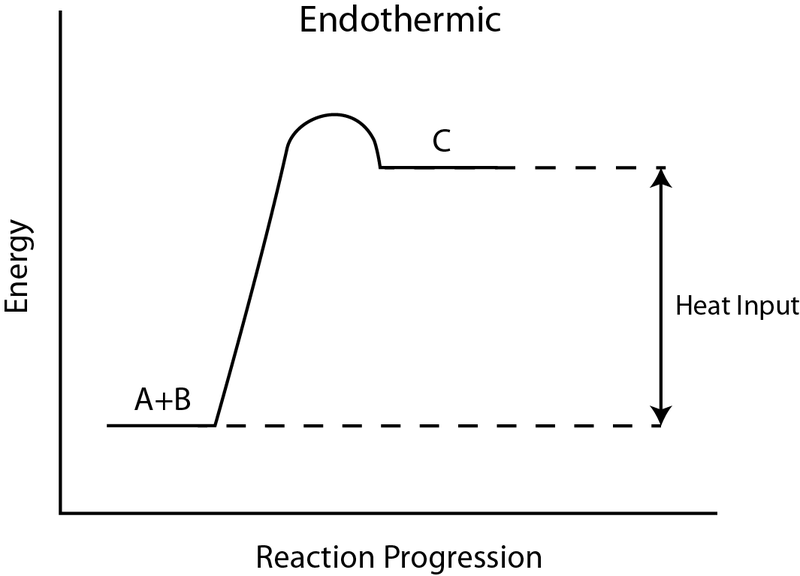
A heat absorption reaction is endothermic. Its enthalpy will be positive, and its surroundings will cool down. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in heat the device emits, the atmosphere may rise in temperature. 09-04-2018 · 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
28 Aug 2021 — From an energy level diagram, we can determine the following: 1. Is the reaction endothermic or exothermic? 2. Does the product have more energy ...
Diagram of exothermic reaction
03-12-2021 · The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a … Endothermic Reaction Diagram | energy diagram for a catalyzed endothermic reaction ... Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited. The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Graph of energy against progress of reaction.
Diagram of exothermic reaction. Energy level diagrams for exothermic reactions. In an exothermic reaction, reactants have more energy than the products. The difference between.8 pages In a chemical equation, the location of the word "heat" can be used to quickly determine whether the reaction is endothermic or exothermic. If heat is released as a product of the reaction, the ... In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other … ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
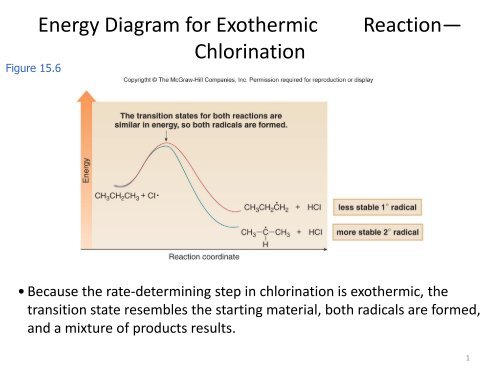
As shown in the energy diagram, the hydrogenation of alkenes is exothermic, and heat is released corresponding to the ΔE (colored green) in the diagram. This heat of reaction can be used to evaluate the thermodynamic stability of alkenes having different numbers of alkyl substituents on the double bond. 01-12-2020 · Applications of exothermic and endothermic reactions in everyday life Application of exothermic and endothermic reactions: The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries. Instant cold packs have separate compartments of water and solid ammonium nitrate placed in a plastic […] Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State … 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ... The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Graph of energy against progress of reaction. Endothermic Reaction Diagram | energy diagram for a catalyzed endothermic reaction ... Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited. 03-12-2021 · The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a …























0 Response to "38 diagram of exothermic reaction"
Post a Comment