38 ground state orbital diagram
Answer (1 of 5): Br electron configuration …. 1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p5 …. or …. [Ar] 3d10, 4s2 4p5 When listed in order of increasing energy, the 4s ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...

Ground state orbital diagram
Delta-v (more known as "change in velocity"), symbolized as ∆v and pronounced delta-vee, as used in spacecraft flight dynamics, is a measure of the impulse per unit of spacecraft mass that is needed to perform a maneuver such as launching from or landing on a planet or moon, or an in-space orbital maneuver.It is a scalar that has the units of speed. This energetic state then serves to activate a substrate molecule to a lower energy triplet state by collisional exothermic energy and spin exchange, returning the sensitizer to its ground state. A variety of useful sensitizers have been identified, and by clicking on the diagram a … When we talk about the ground state electronic configuration firstly, it is important to know the electronic configuration of the element. As shown above, the Vanadium Electron Configuration of the element Vanadium is Ar 3d3 4s2. Therefore, now it is easier to understand the ground state, and the element Vanadium, its ground state is written as the following; [Ar] 3d 3 4s 2. How many must be ...
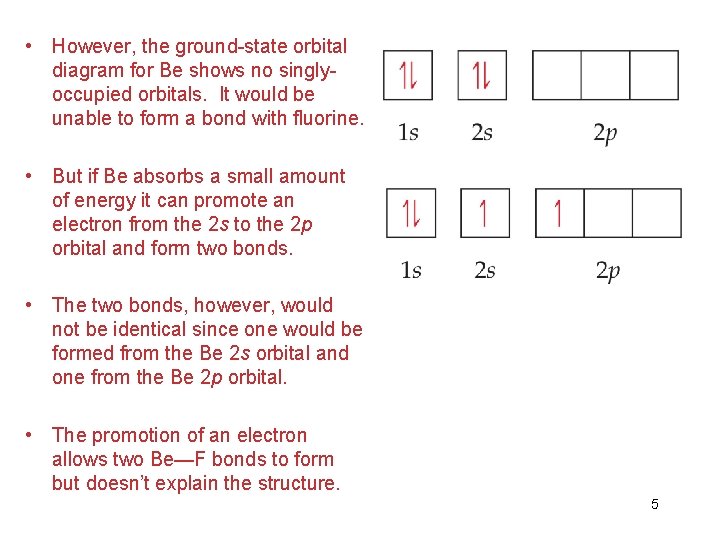
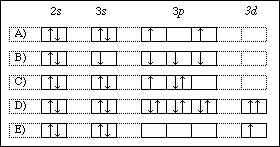
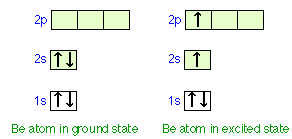
Ground state orbital diagram. Be 's ground-state electron configuration is the. Electron configurations and orbital diagrams can be determined by applying the An atom of the alkaline earth metal beryllium, with an atomic. Since the 2s orbital is completely filled, a new type of orbital must be of one more atom, carbon, with the aid of the color-coded diagrams. Diagram of the nuclear composition, electron configuration ... 18 2.5 points Choose the valence orbital diagram that represents the ground state of Sr2.. A) [Ar] 11 111111 4p B) [Kr] 11 5s 11 5p C) [Kr] 55 44 D) [kr] 11 53 E) [Ar] 10 45 Ο Α B С Ο Ε. Question: 18 2.5 points Choose the valence orbital diagram that represents the ground state of Sr2.. Figure %: The ground state electron configuration of carbon, which has a total of six electrons. The configuration is determined by applying the rules of the Aufbau Principle. Valency and Valence Electrons The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron.
Orbital state energy level: atom/ion with nucleus + one electron. Assume there is one electron in a given atomic orbital in a hydrogen-like atom (ion). The energy of its state is mainly determined by the electrostatic interaction of the (negative) electron with the (positive) nucleus. 8 May 2021 — ... ground state of a neutral carbon atom does indeed contain two unpaired electrons. Exercise 2.2.1. Draw an orbital diagram for nitrogen, ... Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. The following is an orbital diagram for selenium. Oct 15, · Draw the orbital diagram for the ground state of Selenium. Let's put all these stuff into play, how this all come together. Okay let's do the orbital diagram for iron, iron we know is on its ground state of 26 electrons, so we know the first electrons are going to go into the 1s orbital and we said 2 electrons can fall into the 1s orbital.
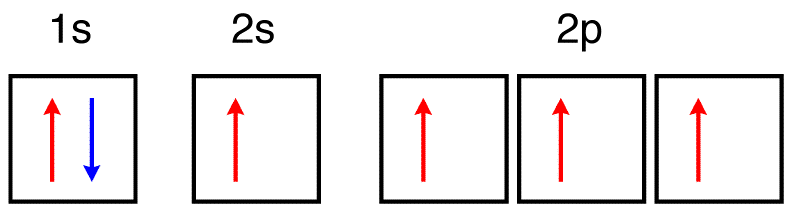
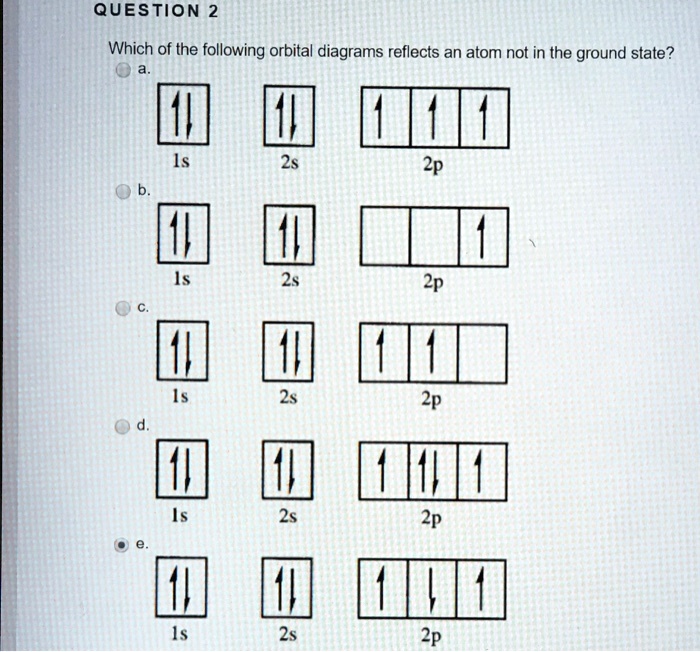
For the orbital diagrams (with the arrows showing the paired/parallel electrons), the ground state electron configuration of an atom would only be the configuration made by the Aufbau principle (and Pauli's/Hund's). Any other configuration (with different numbers of electrons occupying different orbitals) would be the excited state. • The orbital approximation allows us to express the electronic structure of an ... The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. Ground State: 1s22s22p x 12p y 1 (or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1) [CAUTION: these don’t explicitly state the electron’s spin!] CHEM 2060 Lecture 9 ... The ground state is 1s^2 2s^2 2p^2. In the explanation below, I show a common means of diagramming this. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way: The single electrons in the two p-orbitals is in accordance with Hund's Rule. 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
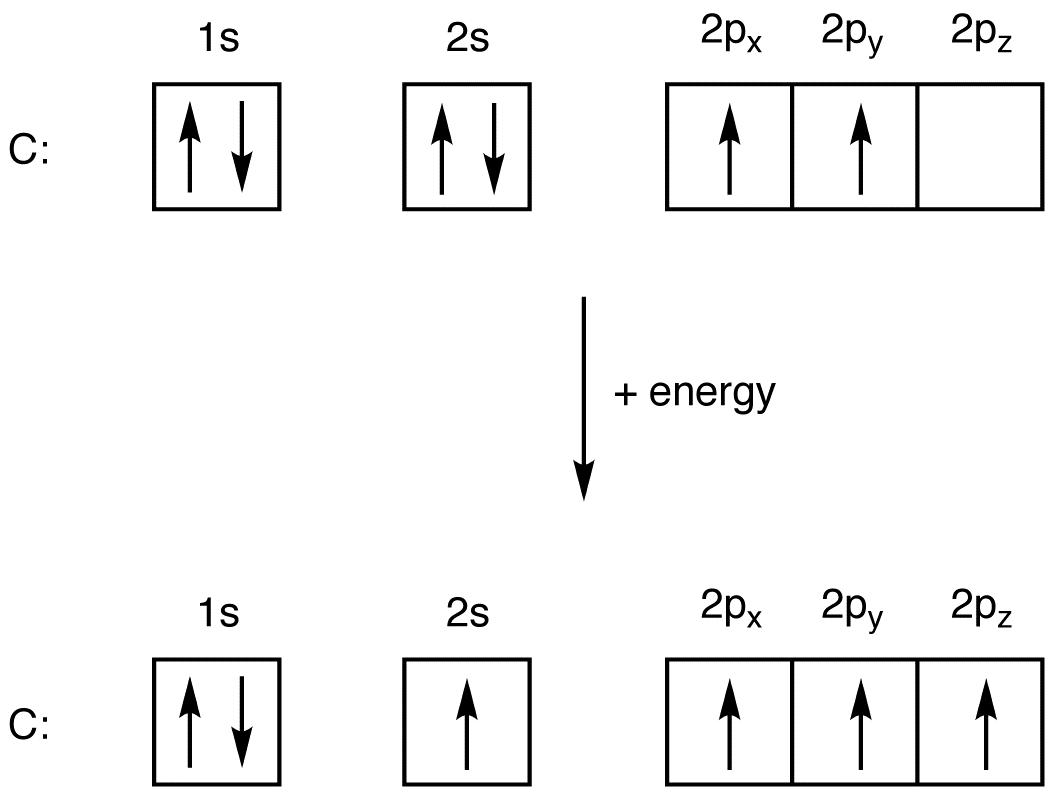
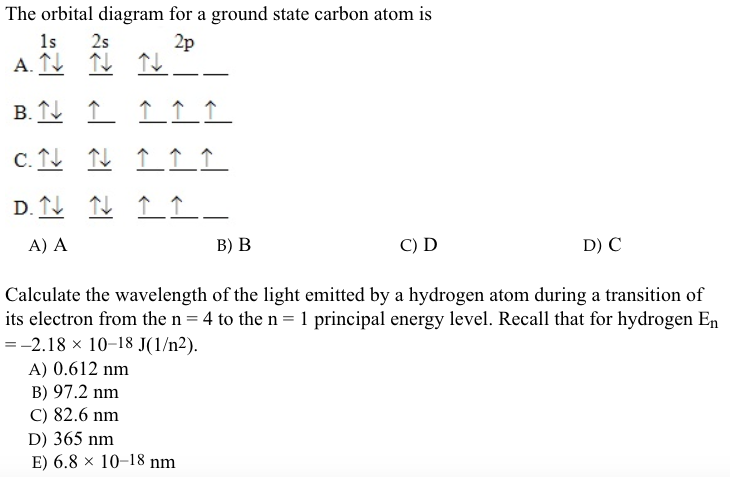
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
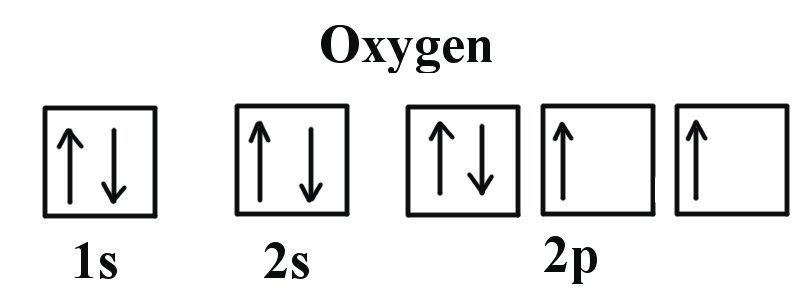
Answer to: The orbital diagram for a ground-state oxygen atom is? By signing up, you'll get thousands of step-by-step solutions to your homework...
The good news is that orbital diagrams, electron configurations (both in shorthand and full form) and dot diagrams for electrons are really easy to understand once you’ve grasped a few basics. TL;DR (Too Long; Didn't Read) Electron configurations have the format: 1s 2 2s 2 2p 6. The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum ...
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating ...
1.9A: Ground state electronic Configuration. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties.
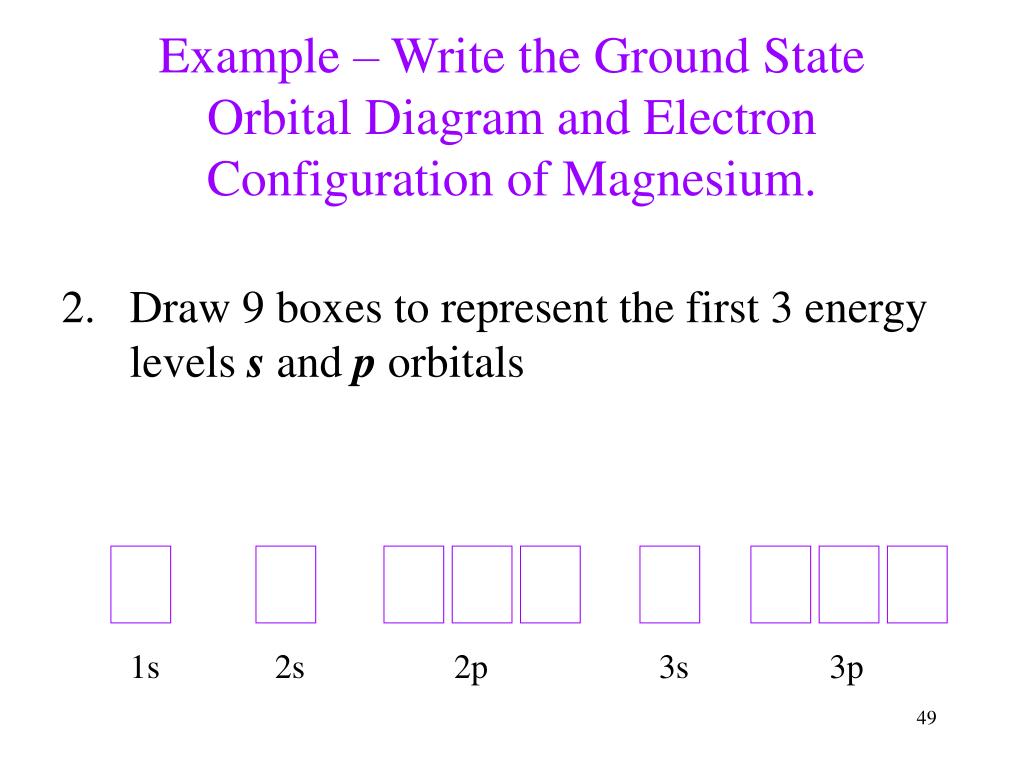
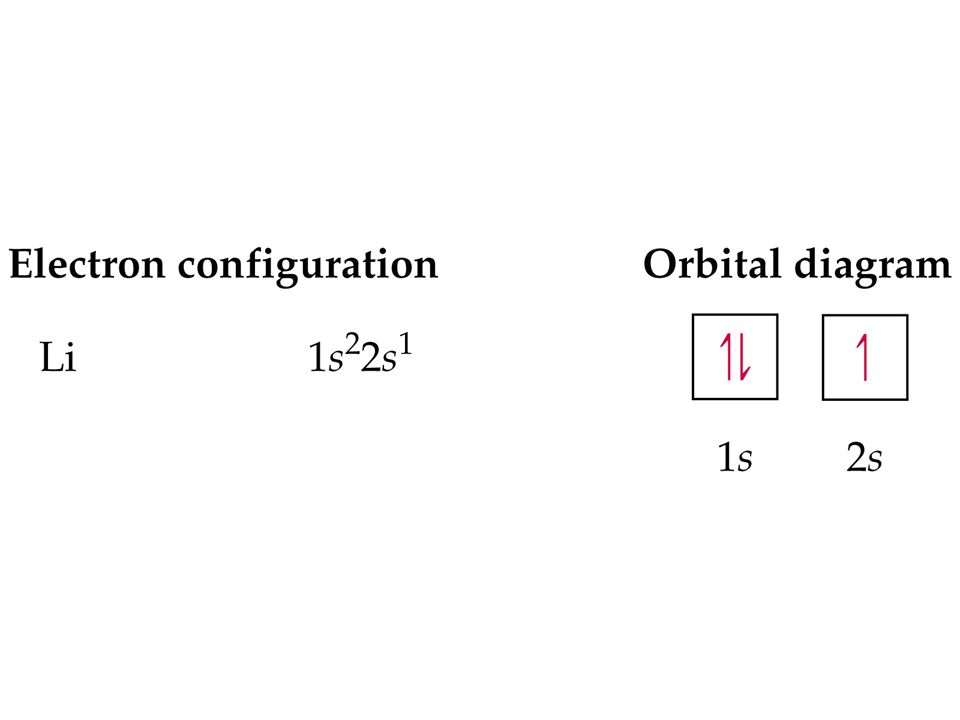
We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons.
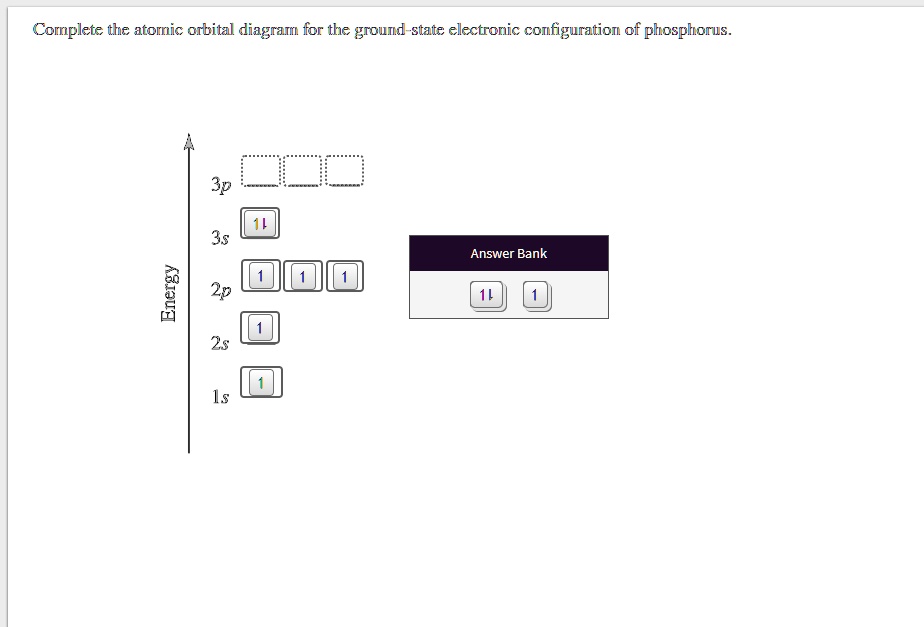
orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the Aufbau principle ("aufbau"means filling up). Examine the ...
Draw an orbital diagram for nitrogen, Z = 7. Which is the configuration of an atom in the ground state? The ground state configuration is the lowest energy, most stable arrangement. An excited state configuration is a higher energy arrangement (it requires energy input to create an excited state).
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3. Below you can get the full image representation which will help you to understand the topic more easily. If you are new here and are more excited to get the full information of the element than here you can get ...
This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl...
Writing Orbital Diagrams. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We ...
From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle.
Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s 2 2s 2 2p 6. Because the second energy level (2s 2 2p 6) has eight electrons Neon has an octet and has a full outer shell. It is therefore a Nobel Gas.
The arrangement of electrons in the atomic orbitals of an atom is called the electron ... orbital energy diagram, a ground state electron configuration ...
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
Satellite Communication Block Diagram. Need for Satellite Communication. We know that there are different ways to communicate and the propagation of these waves can take place in different ways. Ground wave propagation and skywave propagation are the two ways in which communication took place for a certain distance.
Write a ground state electron configuration for each neutral atom. Ground state means that all of the lowest possible energy levels (up to the proper number of electrons for the element) are filled. 1. Na 2. Pb 3. Sr 4. U 5. N 6. Ag 7. Ti 8. Ce 9. Cl 10. Hg Write a ground state electron configuration for these ions.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
State Hund's rule. Apply Hund's rule to the filling of orbitals. Use orbital filling diagrams to describe the locations of electrons in an atom.
When we talk about the ground state electronic configuration firstly, it is important to know the electronic configuration of the element. As shown above, the Vanadium Electron Configuration of the element Vanadium is Ar 3d3 4s2. Therefore, now it is easier to understand the ground state, and the element Vanadium, its ground state is written as the following; [Ar] 3d 3 4s 2. How many must be ...
This energetic state then serves to activate a substrate molecule to a lower energy triplet state by collisional exothermic energy and spin exchange, returning the sensitizer to its ground state. A variety of useful sensitizers have been identified, and by clicking on the diagram a …
Delta-v (more known as "change in velocity"), symbolized as ∆v and pronounced delta-vee, as used in spacecraft flight dynamics, is a measure of the impulse per unit of spacecraft mass that is needed to perform a maneuver such as launching from or landing on a planet or moon, or an in-space orbital maneuver.It is a scalar that has the units of speed.






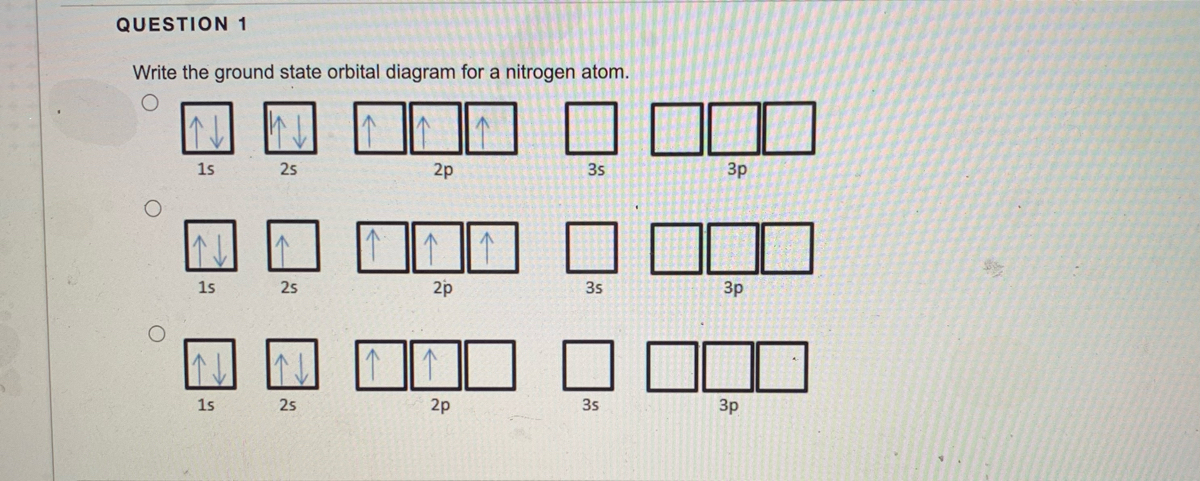








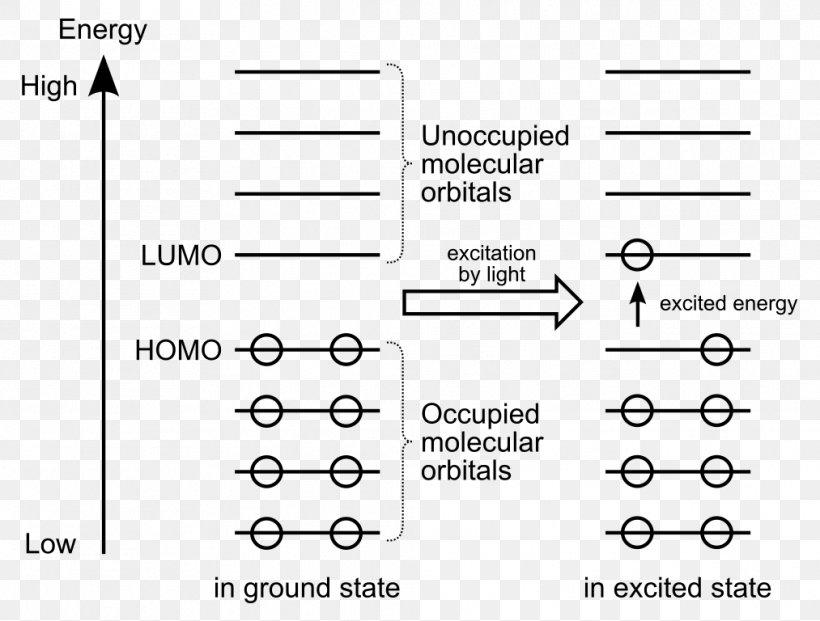




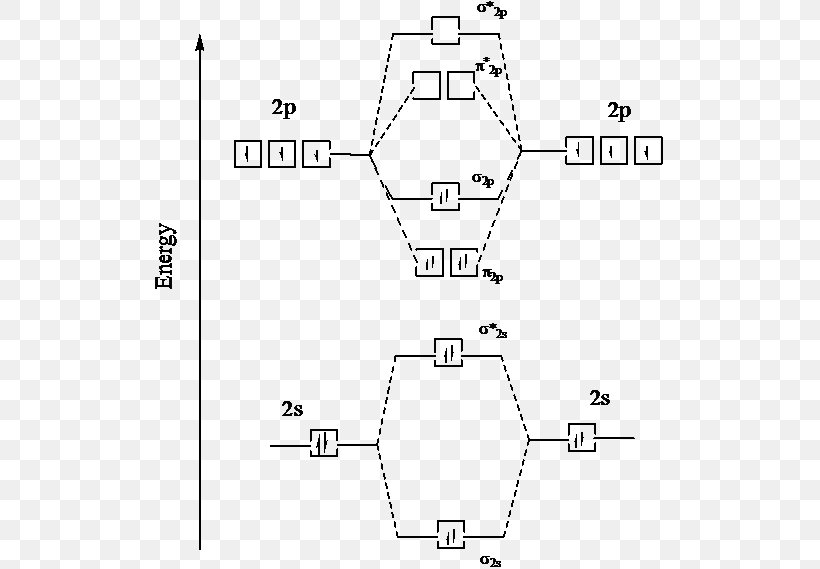

0 Response to "38 ground state orbital diagram"
Post a Comment