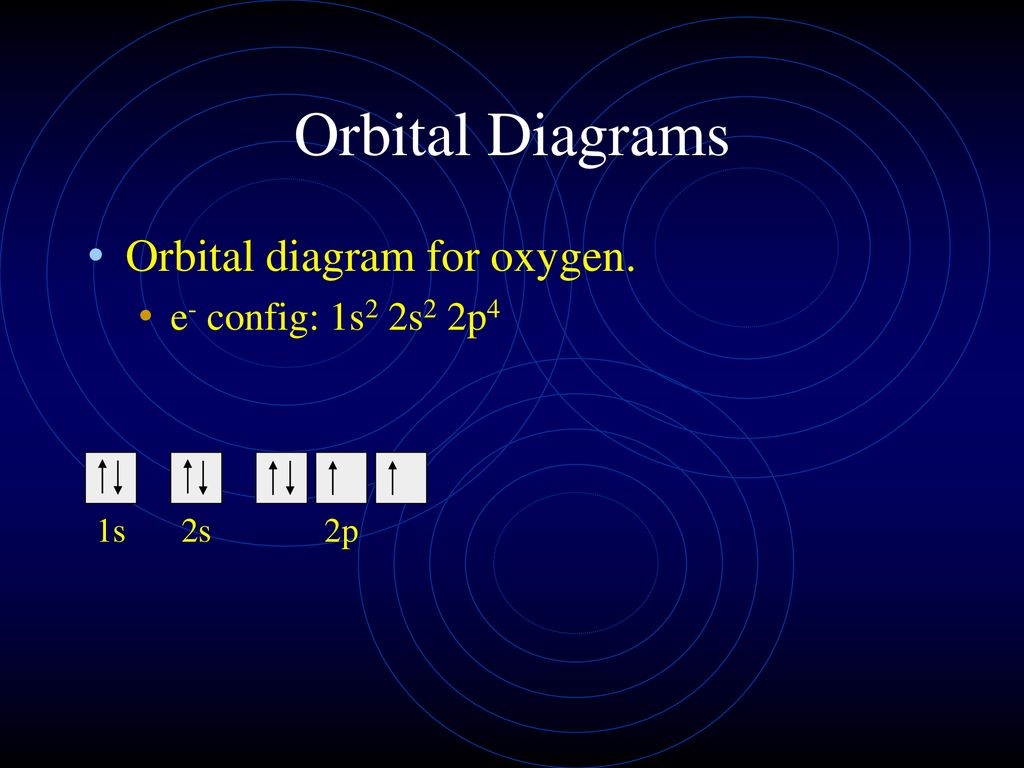
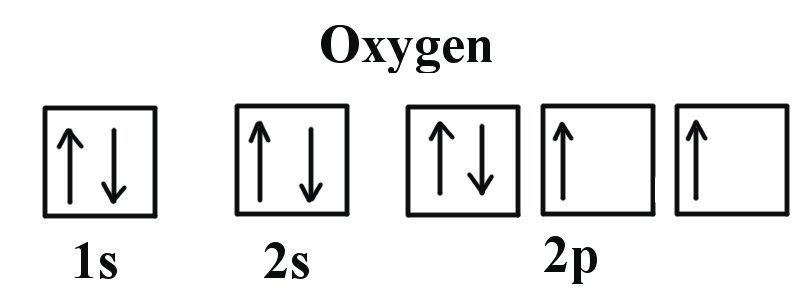
39 orbital diagram of oxygen
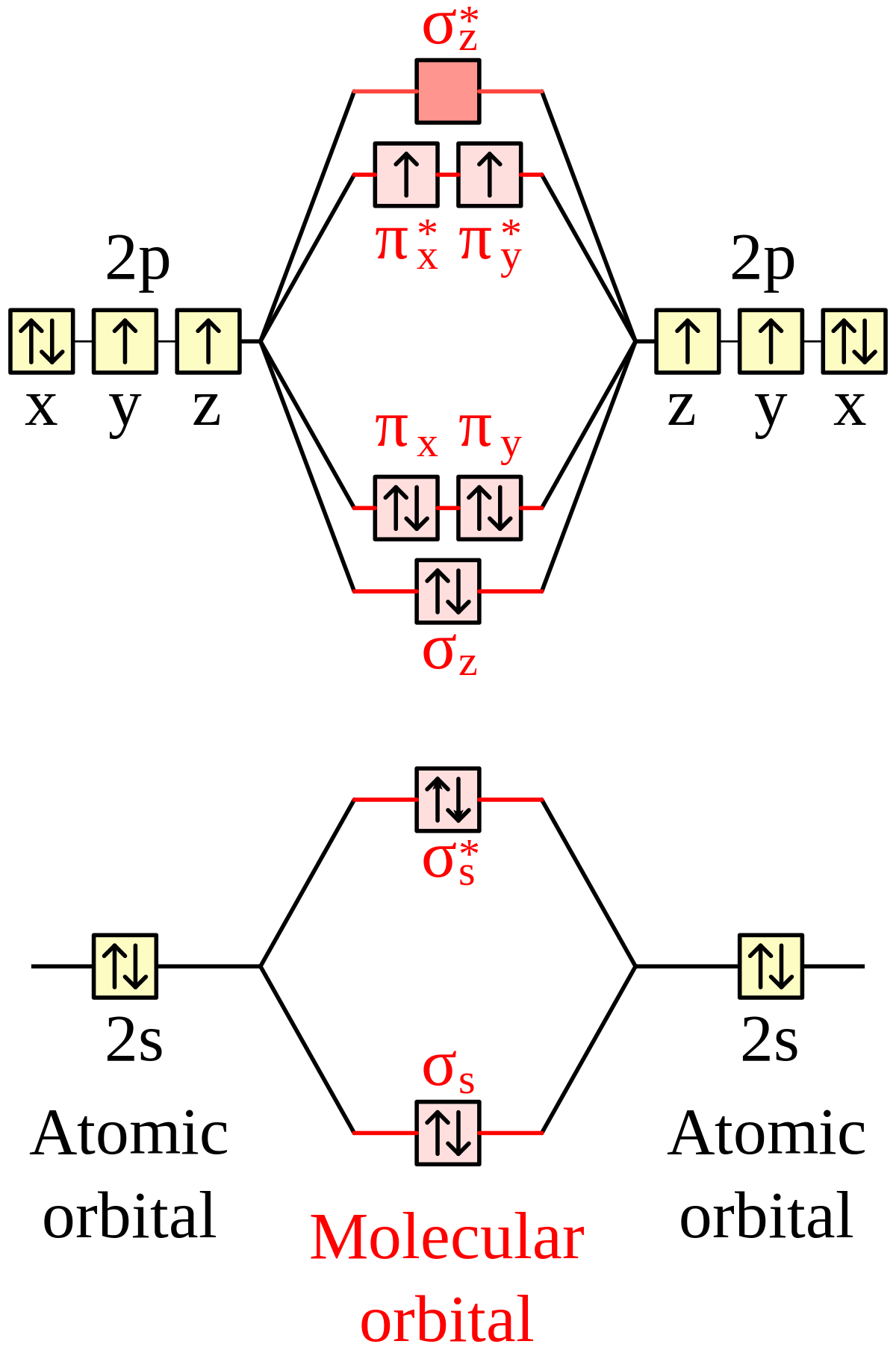
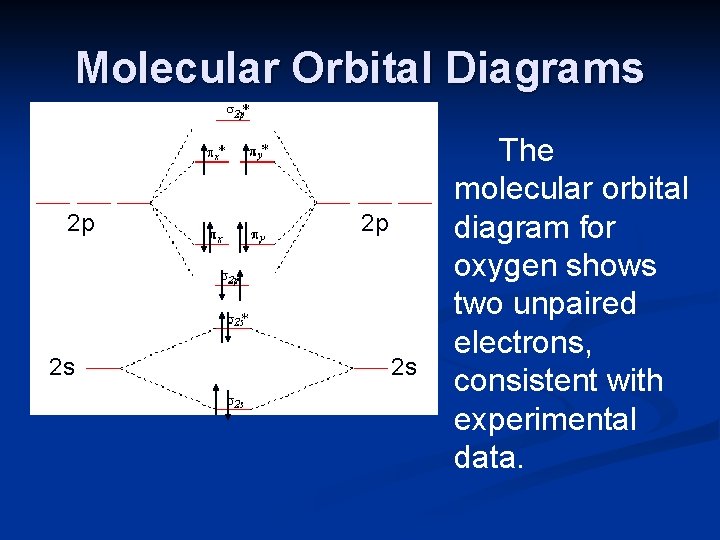
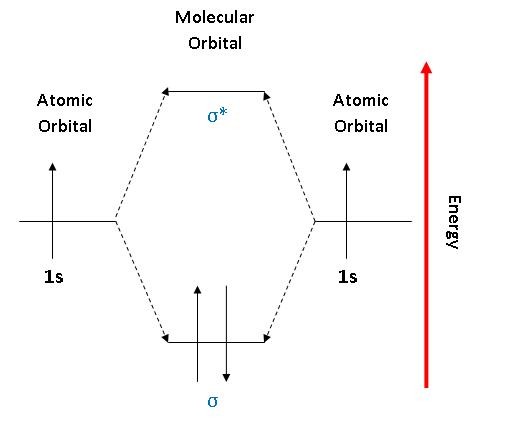
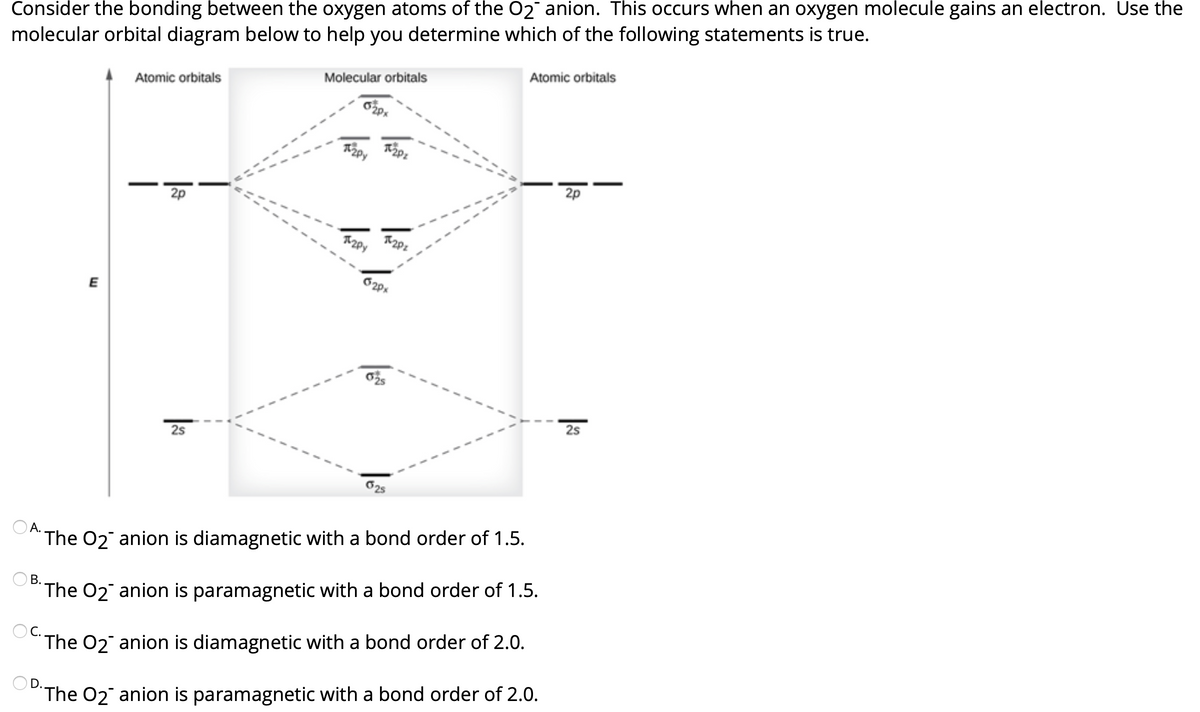
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
Which orbital-filling diagram represents the ground state of oxygen? A. [ [He] 신 N 2p 치어 B. [He] 신 소 소 2p 25 C. [He] 个个 2s 신 소 소 2p D. [He] _ 28 솨 신 소 2p 치 ; Question: Which orbital-filling diagram represents the ground state of oxygen? A.
Orbital diagram of oxygen
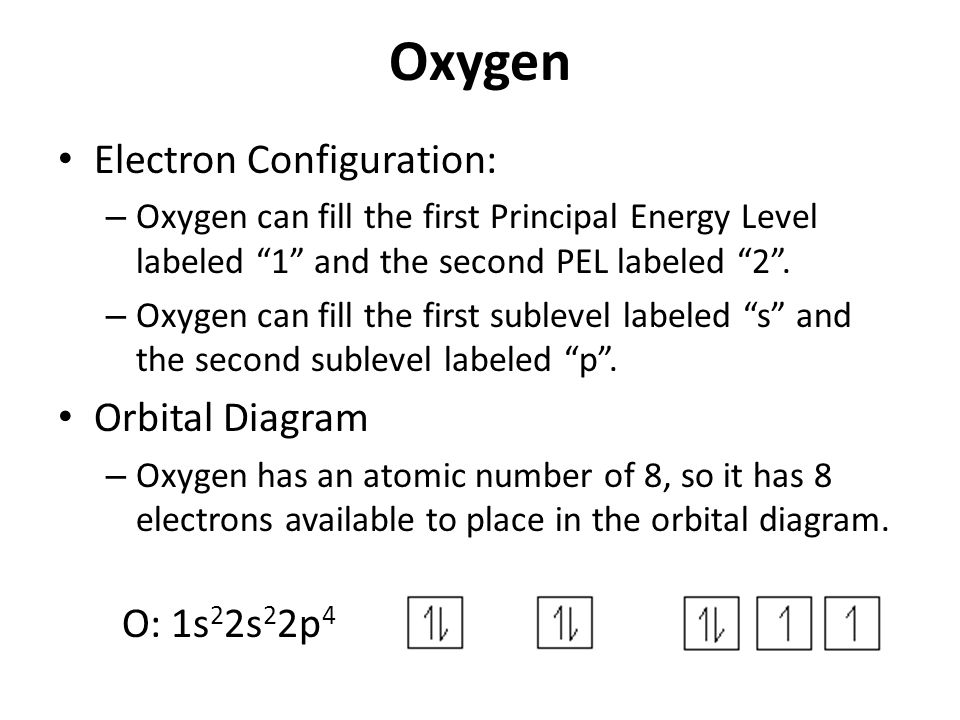
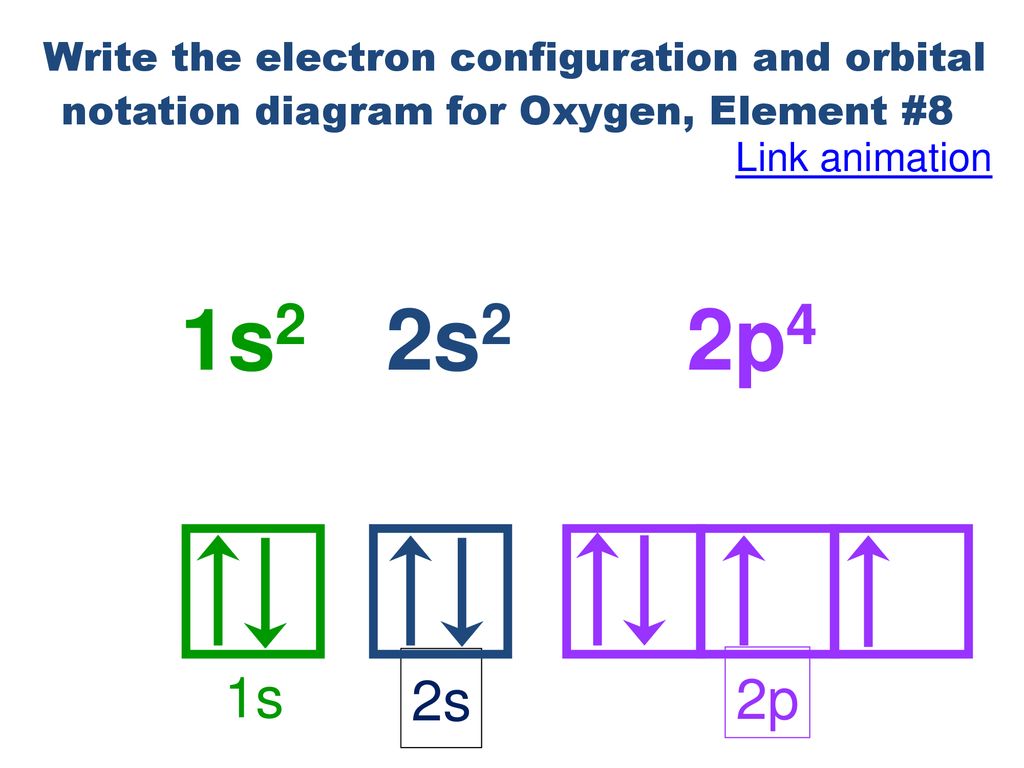
Oct 28, 2021 · Fig. 1: Brain organoid generated by vertical mixing showed inverted structure in comparison with brain organoid generated by orbital mixing. a Schematic diagram of conditions used to induce brain ... Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
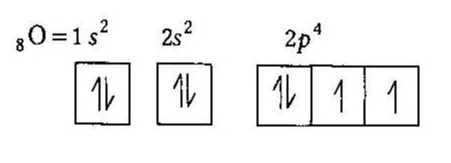
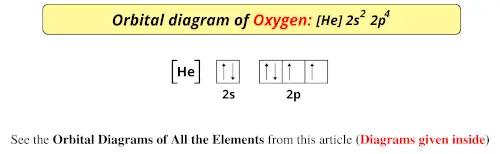
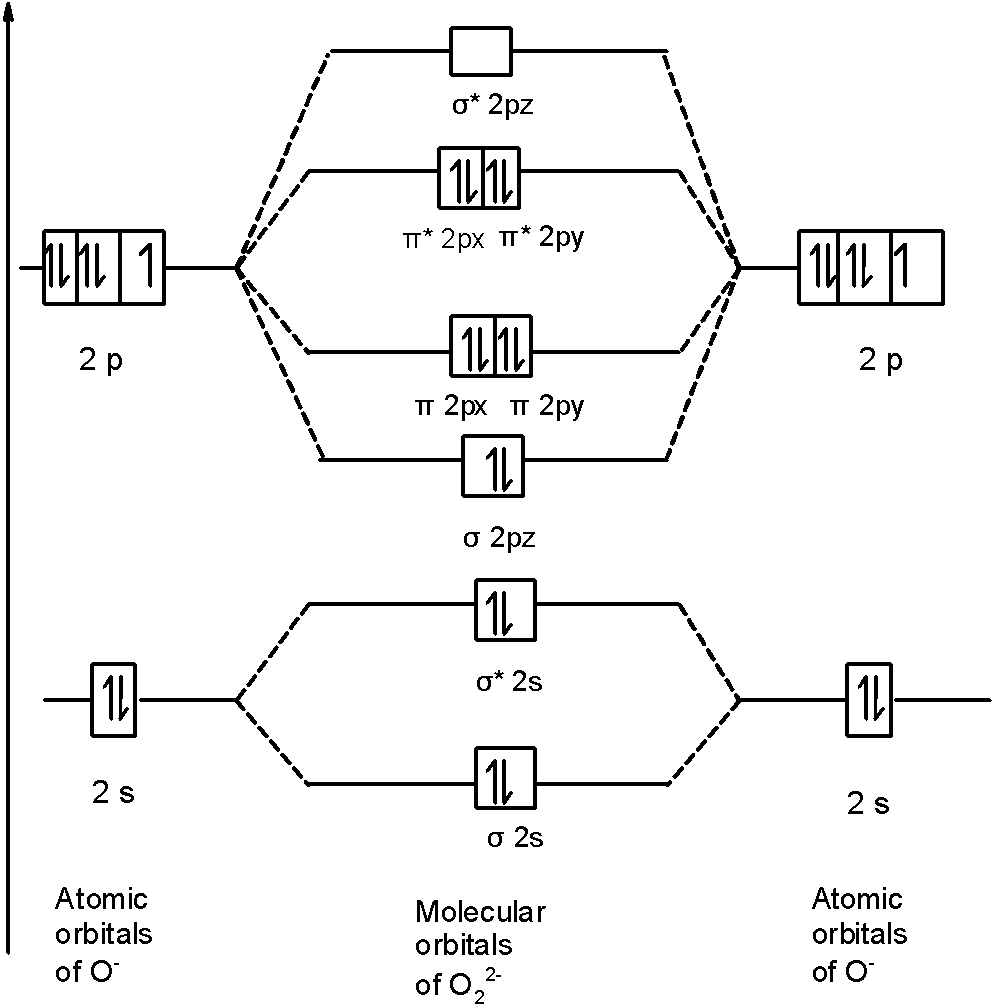
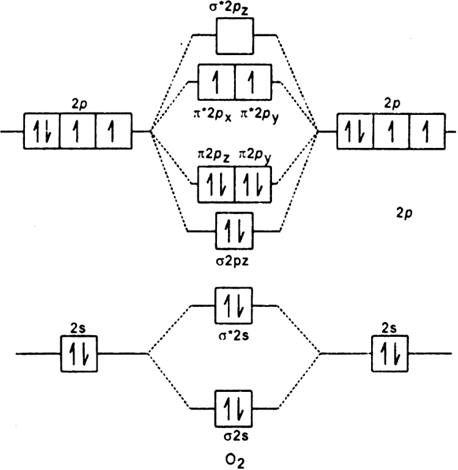
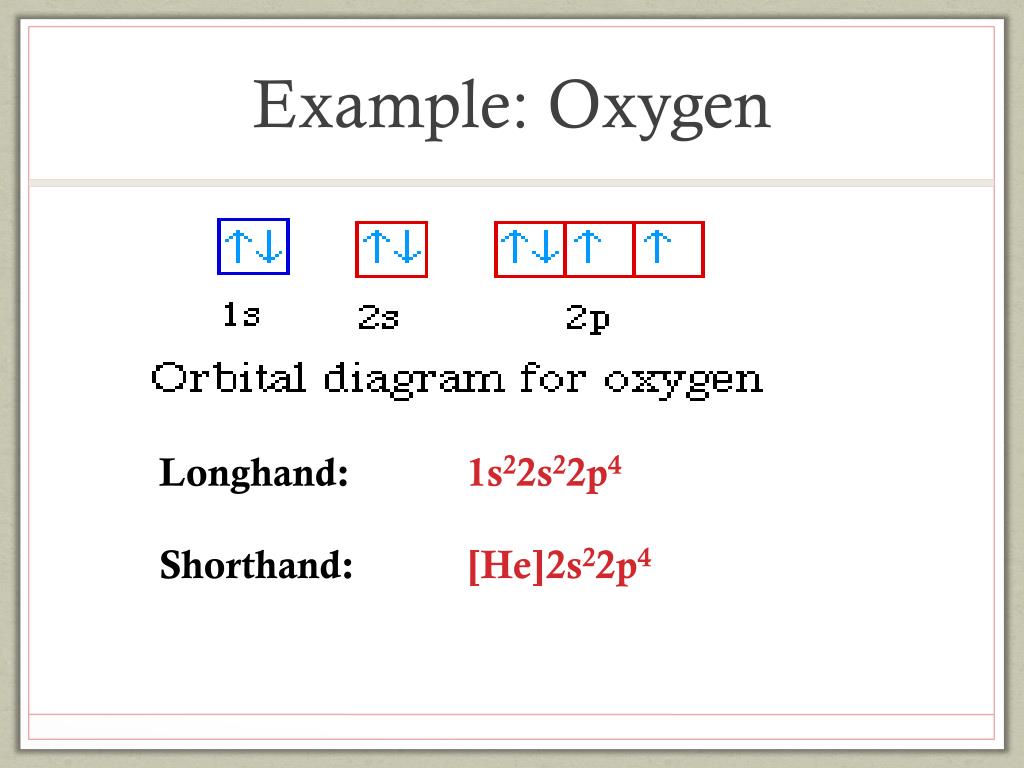
Orbital diagram of oxygen. Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11. 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... MO diagrams explain why some molecules exist and others do not. By looking at the O2 molecular orbital diagram, we can see that oxygen has BO of 2 because it has 10 bonding and 6 anti-bonding. Experiments show that each O2 molecule has two unpaired electrons. We can also notice the magnetic property of diamagnetic or paramagnetic.
In carbon dioxide molecule, oxygen also hybridizes its orbital s to form three sp 2 hybrid orbital s. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond. However, out of the three sp hybrid orbital s, only one will be used to form a bond with the carbon atom. Part B. Draw the orbital diagram for the ion Co2+.Use the buttons at the top of the tool to add orbital s in ... A scramjet (supersonic combustion ramjet) is a variant of a ramjet airbreathing jet engine in which combustion takes place in supersonic airflow.As in ramjets, a scramjet relies on high vehicle speed to compress the incoming air forcefully before combustion (hence ramjet), but whereas a ramjet decelerates the air to subsonic velocities before combustion using shock cones, a scramjet has no ... Definition of atomic orbital diagram for oxygen: An orbital is the region of space around the nucleus within which the probability finding an electron of given energy is maximum. The diagram of this region gives the diagram of the orbital. The plot of angular wave function or square of angular wave functions give us the diagram of orbitals. As bond order in Oxygen is 2 so two bonds i.e. double bond is formed between two oxygen atoms (O=O). Further more as there are two unpaired electrons in Oxygen molecule hence it is paramagnetic. Also Watch Molecular orbital diagram of O2 , O2 +2, 02 - 2 ( in Urdu / Hindi) Simplest Trick to Calculate Bond Order :
And it shows you what the molecular orbital mo diagram for o2 is. Complete this valence molecular orbital diagram for oxygen o2. Click the blue boxes to add electrons as needed. Complete these structures by adding bonds and lone pairs as necessary. Apr 25, 2018 · You'll need the molecular orbital (MO) diagram of O2. Begin with the atomic orbitals. Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ... Explanation: Typically, an atom of O has 8 electrons, so based on the electron configuration system that would be 1s22s22p4, or 2 + 2 + 4 = 8e−. However, O+ means you've lost an electron, hence the positive charge. Thus, you subtract an electron: Definition of atomic orbital diagram for oxygen: An orbital is the region of space around the nucleus within which the probability finding an electron of given energy is maximum. The diagram of this region gives the diagram of the orbital. The plot of angular wave function or square of angular wave functions give us the…
The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example: Looking at the periodic table, you can see that Oxygen has 8 electrons.
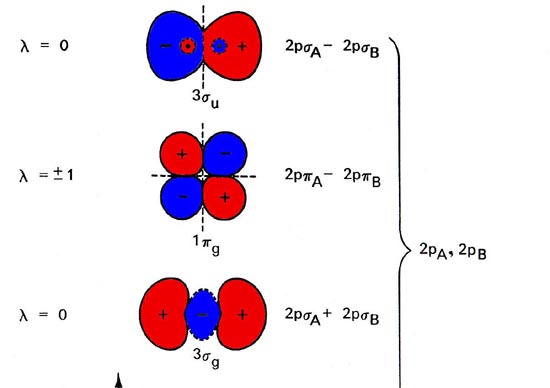
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
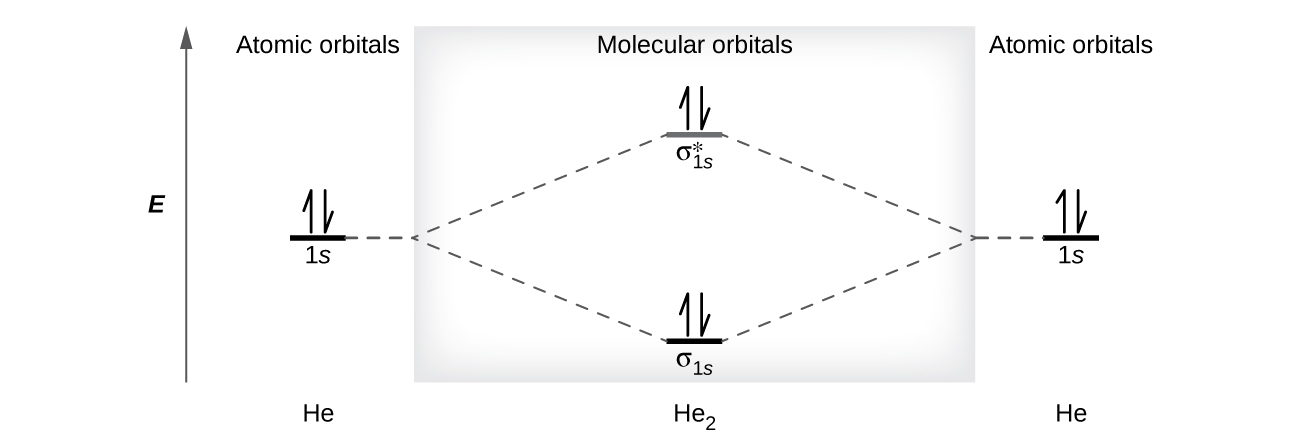
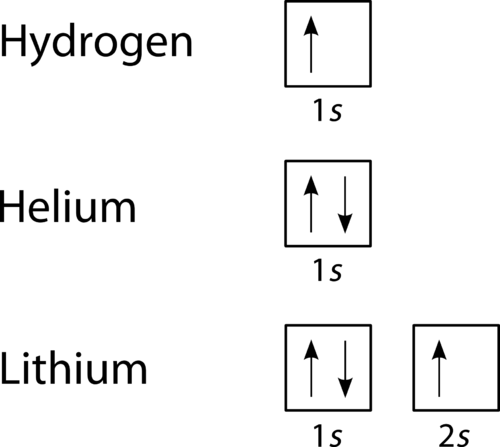
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Draw the orbital diagram for the electron configuration of oxygen, and determine the four quantum numbers for each electron. Question: Draw the orbital diagram for the electron configuration of oxygen, and determine the four quantum numbers for each electron.
The molecular orbital electronic configuration of hydrogen molecule is (s 1s) 2. The molecular orbital energy level diagram of H 2 molecule is given in Fig.. The bond order of H 2 molecule can be calculated as follows. Here, N b = 2 and N a = 0. Bond order = (N b -N a) /2 = 2-0/2 = 2 i.
Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
MO Diagram of SF2. MO diagram s are a good way to represent the different properties of a compound. These properties include shape, bond energy, bond angle, and more such things. With the help of this diagram, we can showcase the energy that different energy orbital acquires and have. Electronic configuration of Oxygen atom is.
Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Question: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.
is interesting to examine the energy diagram of the oxygen atom because similarities with the energy diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. ... However, the first excited state of atomic oxygen (1D) has one 2p empty orbital. In this case, the 1D ...
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
oxygen for the magnetic field demonstrates the paramagnetism of the O2 molecule. Figure 9.41: The molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial
Draw the valence shell molecular orbital diagram of the oxygen molecule and predict its magnetic nature. Answer. Verified. 109.2k+ views. Hint: The magnetic property of a molecule can be explained based on the molecular orbital theory. The molecule which does not contain the unpaired electron is known as the paramagnetic. The molecule which has ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen.
Oct 28, 2021 · Fig. 1: Brain organoid generated by vertical mixing showed inverted structure in comparison with brain organoid generated by orbital mixing. a Schematic diagram of conditions used to induce brain ...














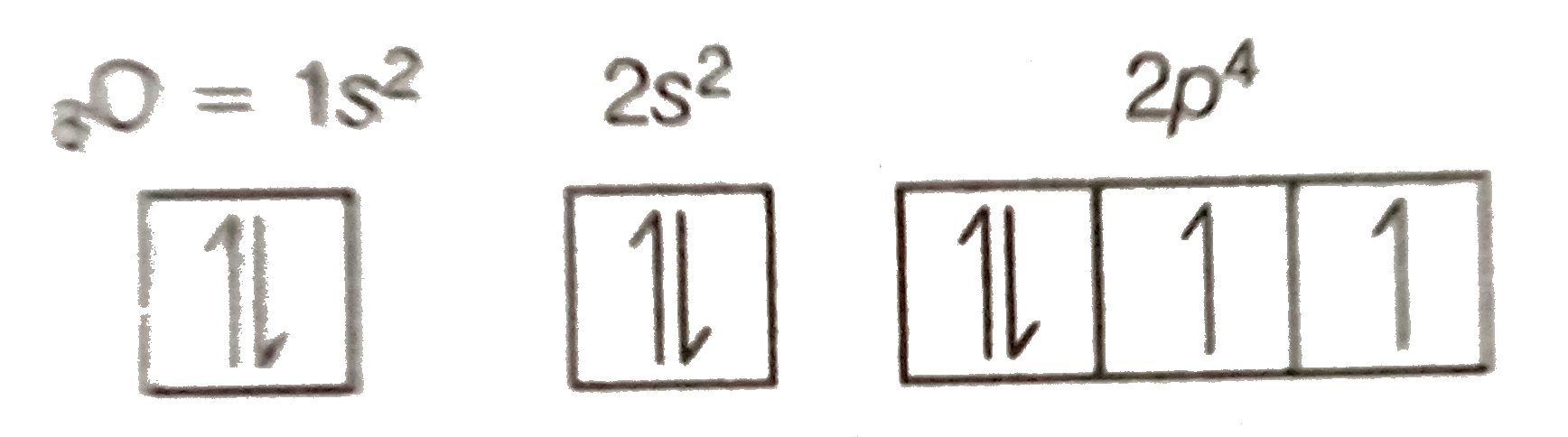



0 Response to "39 orbital diagram of oxygen"
Post a Comment