39 which diagram shows a pair of electrons that have opposite spins?
Which diagram shows a pair of electrons that have opposite spins? A box contains an upward pointing and a downward pointing arrow. A box contains an upward pointing arrow.A box contains a downward pointing arrow. A box contains an upward pointing arrow.A box contains an upward pointing arrow. A box contains two upward pointing arrows.
High School. answer. answered. Which diagram shows a pair of electrons that have opposite spins? O1. 1. 个个. 1. See answer.
Which diagram shows a pair of electrons that have opposite spins? O1 1 个个 - DocumenTV. Which diagram shows a pair of electrons that have opposite spins? O1 1 个个. Which diagram shows a pair of electrons that have opposite spins?

Which diagram shows a pair of electrons that have opposite spins?
The correct answer on edgenuit.y is A) updown the arrows that are combined together. Im sorry im late but I hope this helps anyone else . The answer will be a The pair with opposite spins is up down The spins of electrons are designated using arrows and opposite spins are designated by arrows facing in the opposite directions.
*The electrons of a shared pair must have opposite spins ... - The MO diagram shows the relative energy and number of electron in each MO. ... A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular ...
The use of the above diagram of the virtual particle producing a quark–antiquark pair was featured in the television sit-com The Big Bang Theory, in the episode "The Bat Jar Conjecture". PhD Comics of January 11, 2012, shows Feynman diagrams that visualize and describe quantum academic interactions , i.e. the paths followed by Ph.D. students ...
Which diagram shows a pair of electrons that have opposite spins?.
Which diagram shows a pair of electrons that have opposite spins? A box contains an upward pointing and a downward pointing arro ... Because they have a limited of thing so they have to reuse resources. 3 0. 8 months ago. Other questions: ... Electron dot diagram for four elements are shown. Which element would be found in Group 13? 13 ...
molecular orbital diagram as in diboron these two unpaired electrons have the same spin in the ground state which is a paramagnetic diradical triplet oxygen the first excited state has both homo electrons paired in one orbital with opposite spins and is known as singlet oxygen
06.11.2021 · Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital …
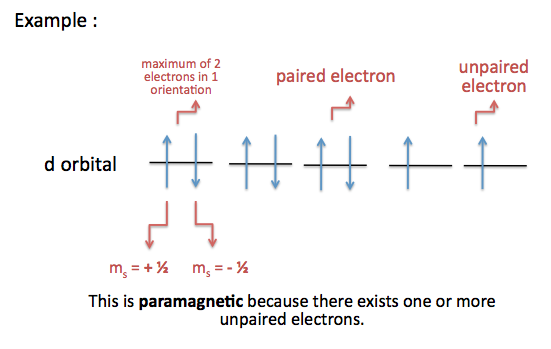
As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins. This means if one is assigned an up-spin ( +½), the other must be down-spin (-½). An explanation of this is that an electron has a magnetic field due to its spin.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
-The two electrons in the 2s sublevel have opposite spin. When drawing an orbital diagram, orbitals of _____ energy are filled first. By convention, the _____ electron in a given orbital is designated as ↑ and the direction of the arrow indicates the electron _____.
Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ ...
For the second rule, unpaired electrons in singly occupied orbitals have the same spins. If all electrons are orbiting in the same direction, they meet less often than if some of them orbit in opposite directions. In the latter case, the repulsive force increases, which separates electrons. Therefore, spins that are aligned have lower energy.
Which diagram shows the correct distribution of electrons in the electron shells of a helium atom? c. Which diagram shows a pair of electrons that have opposite spins? a. Which is the electron configuration for lithium? 1s^2 2s^1. Consider the model of a sodium atom.
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
In chemistry an electron pair or a lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Orbital notation shows the spin of the electrons. So person 1 looks at theirs and sees its an upward spin. Each orbital can host two electrons of opposite spin. This collapses person 2s to downward imediantly.
Which diagram shows a pair of electrons that have opposite spins? A To show the electron configuration for an atom, what is the advantage of using an orbital notation compared to a dot structure?
Electrons in higher energy levels (e.g. higher n numbers) have a wider range of l values to choose from. L can be any integer number between n‐1 and 0. For example, an electron in n = 4 could have an l value of 3, 2, 1 or 0. But there is a better way to think about l.
Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron.
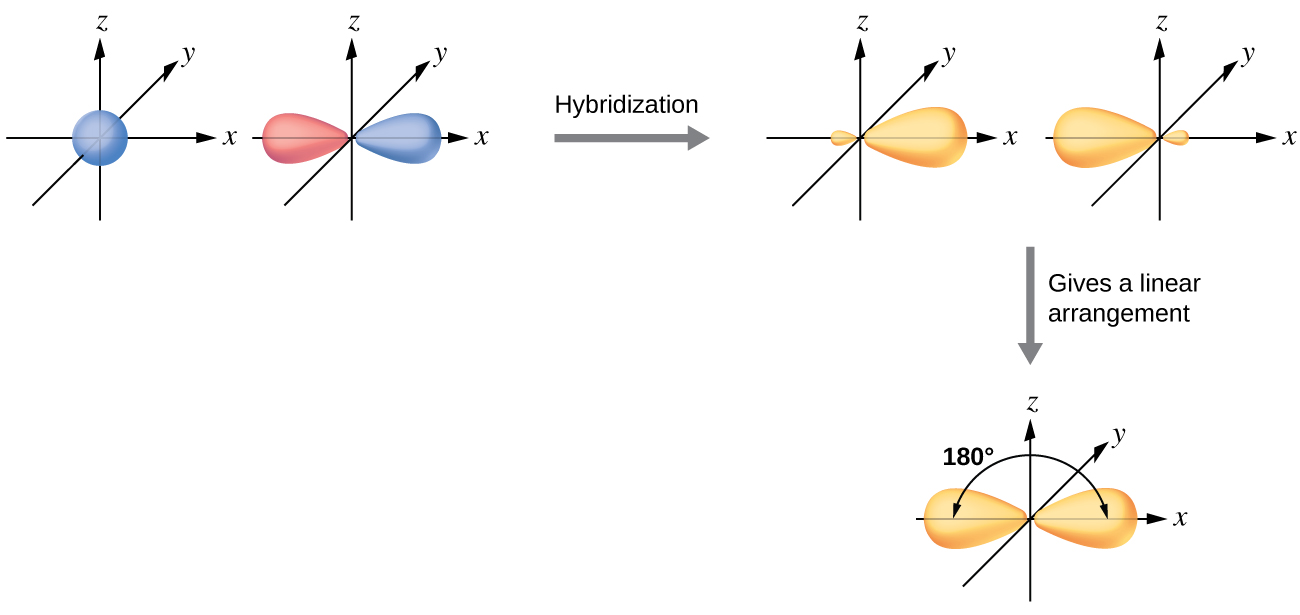
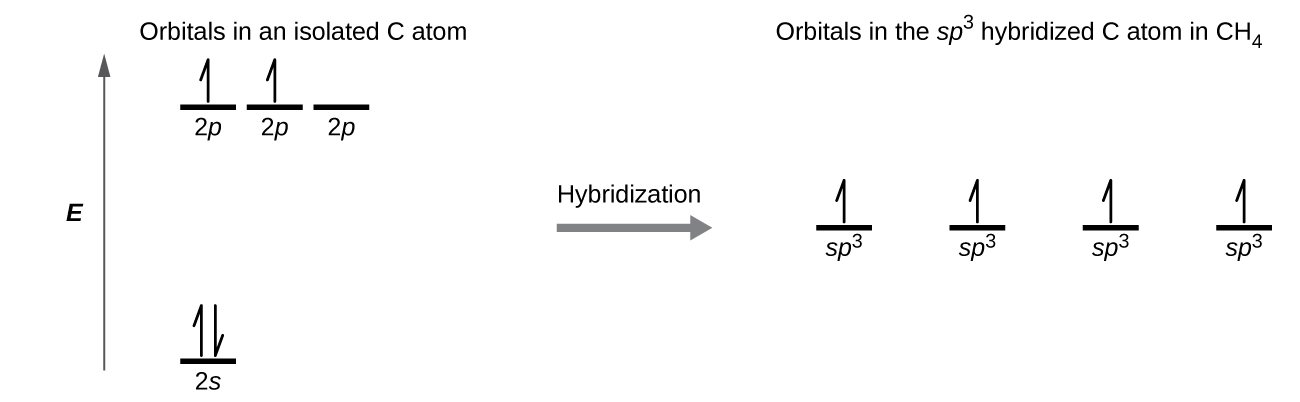




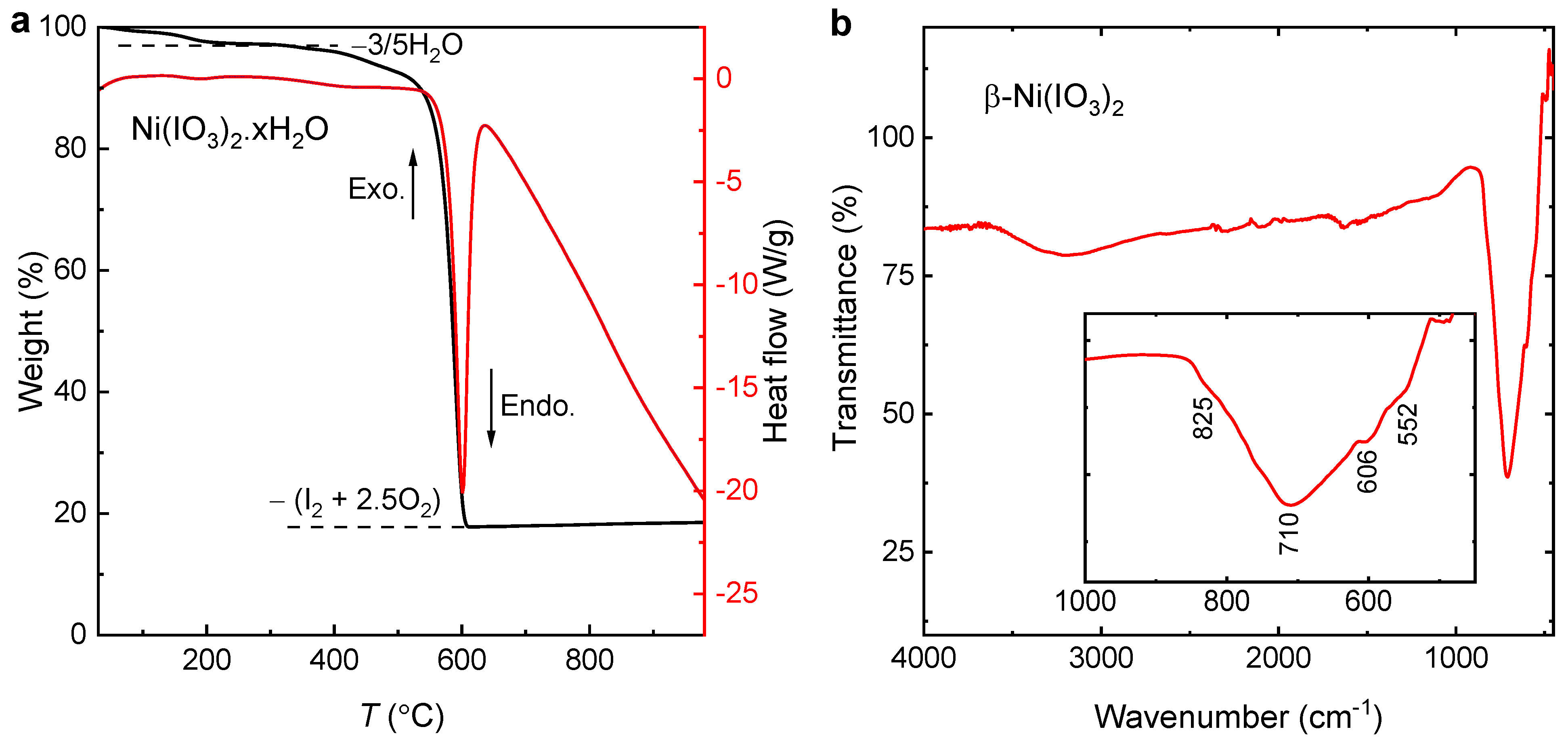
















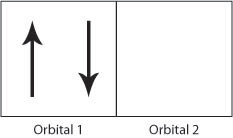




0 Response to "39 which diagram shows a pair of electrons that have opposite spins?"
Post a Comment