40 hydrogen energy level diagram colors
A Hydrogen atom has one proton and one electron. A classic and probably more familiar model for the Hydrogen atom looks like this The nucleus in red is a single proton, and the electron in yellow...
Hence, the photocatalytic hydrogen production reaction has very high requirements on the catalyst materials. It not only needs to have a suitable energy-level structure to absorb enough visible light and generate electron-hole pairs, but more importantly, it must effectively separate and transport photogenerated electrons and holes.
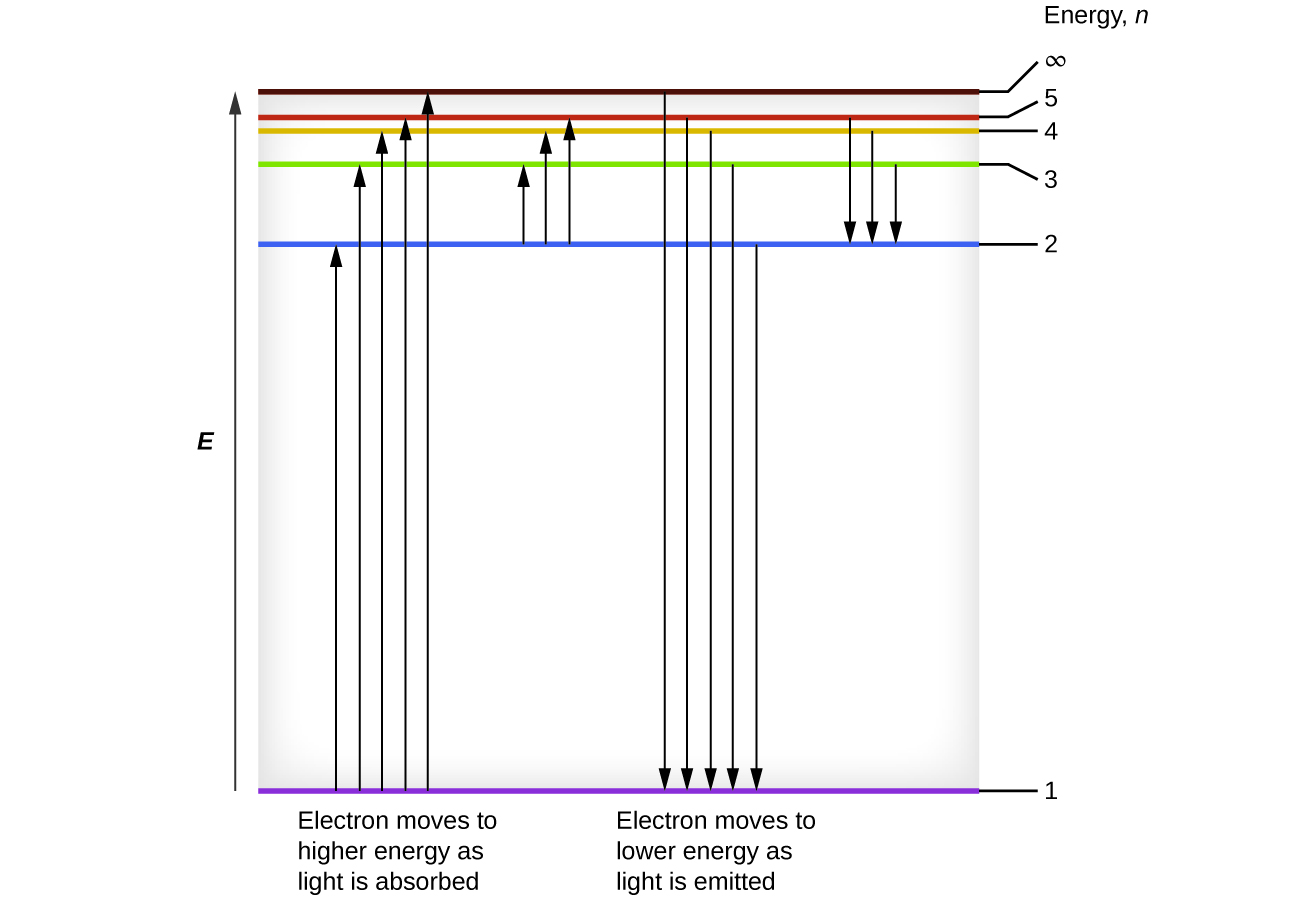
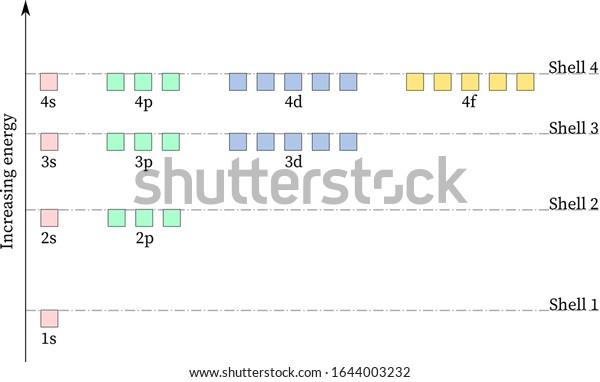
Atoms, ions and molecules have defined energy levels associated with energy levels that electrons in the matter can hold. Light can be generated by the matter or a photon of light can interact with the energy levels in different ways. Properties of light can be represented in energy level diagrams also called Jablonski diagrams.
Hydrogen energy level diagram colors
We report on an electron-spin resonance study of nuclear-polarized phases of hydrogen atoms embedded in solid H $$_{2}$$ 2 films at temperatures 0.1-1.5 K and a high magnetic field of 4.6 T. The H atoms in solid H $$_{2}$$ 2 were generated by two different dissociation techniques: a cryogenic rf-discharge and bombardment by 5.7 keV electrons via tritium decay. In both studies, we observed a ...
Sergei Winogradsky. Source.. The Winogradsky column is a simple device for culturing a large diversity of microorganisms. Invented in the 1880s by Sergei Winogradsky, the device is a column of pond mud and water mixed with a carbon source such as newspaper (containing cellulose), blackened marshmallows or egg-shells (containing calcium carbonate), and a sulfur source such as gypsum (calcium ...
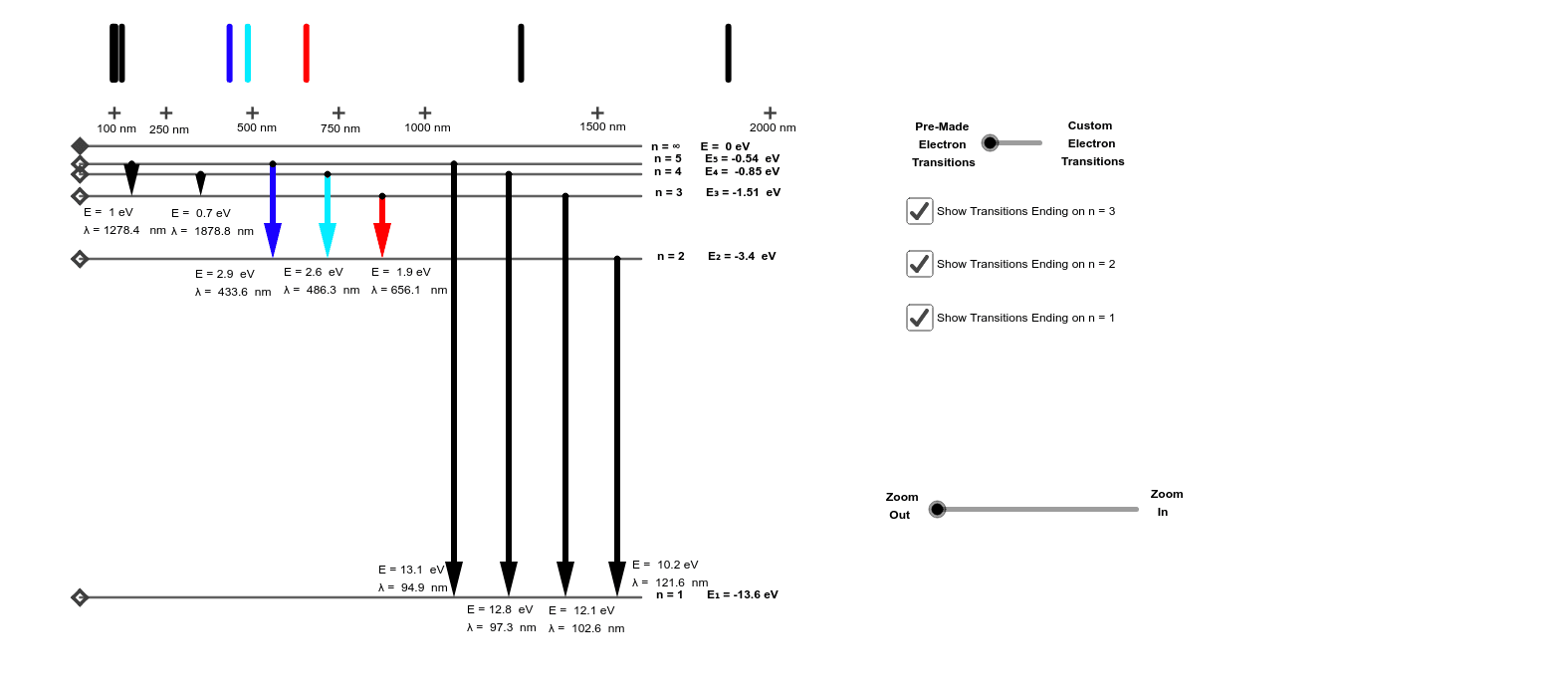
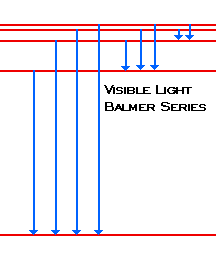
Blueish green color. Balmer. is the name given to a series of spectral emission lines of the hydrogen atom that result from electron transitions from higher levels down to the energy level with principal quantum number 2.
Hydrogen energy level diagram colors.
(D) Electron energy radius on Bohr model of hydrogen atom. (E and F) Energy levels for identified atomic lines in (A). Credit: Science Advances, doi: 10.1126/sciadv.abj0349
Chloroplasts Capture Sunlight. Every second, the sun fuses over 600 million tons of hydrogen into 596 tons of helium, converting over 4 tons of helium (4.3 billion kg) into light and heat energy. Countless tiny packets of that light energy travel 93 million miles (150 million km) through space, and about 1% of the light which reaches the Earth's surface participates in photosynthesis.
Remember those colors of the rainbow - red, orange, yellow, green, blue and violet? Those are listed in the order of increasing energy. Essentially, each transition that this hydrogen electron...
The blue and red dotted lines are hydrogen bonds. while the right one is in the same plane as the fused rings. However, the amino group tends to be planar after the other amino group is functionalized to the nitroimine ( Fig. 2 e) or nitroamine anion ( Fig. 2 f).
Fig. 1 Design strategy of host matrices with different weaving modes. (a) Weaving modes of a quadruple system featuring a skeleton substituted with four hydrogen-bond donors. Two plain weave models are borrowed to illustrate the correlation between hydrogen-bond donor arrangements and intermolecular hydrogen-bond (IHB) network formation: (I) four hydrogen-bond donors (red dot, corresponding to ...
Carbon and hydrogen atoms are shown in gray and white, respectively. (b) Energy level diagram of the pentacene anion showing the electron detachment threshold E th and the electronic excited state below E th. Reuse & Permissions
Light energy initiates the process of photosynthesis when pigments absorb the light. Organic pigments, whether in the human retina or the chloroplast thylakoid, have a narrow range of energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to a populatable ...
Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. This is a 2-D representation and it helps us to understand more about the properties of the compound. Let's move step-by-step and see how the Lewis Structure of C2H5OH can be made. Step 1: Finding the valence electrons for each atom.
Hydrogen combustion is the process by which hydrogen reacts with an oxidizing agent and burns. Hydrogen combustion is an exothermic combustion, meaning that it releases heat energy. Endothermic processes, on the other hand, absorb energy. Hydrogen burns based on the chemical formula 2H 2 + O 2 → 2H 2 O, meaning that it reacts with oxygen.
MO diagram depicts chemical and physical traits of a molecule like bond length, bond energy, bond angle, shape, etc. Following are the steps to design the MO diagram of PCl5 : Step 1: Identify the valence electrons of each atom. In PCl5, it is 5 for P and 7 for every 5 atoms of Cl. Step 2: Check if the molecule is heteronuclear or homonuclear.
To obtain the molecular orbital energy-level diagram for O 2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in part (b) in Figure 6.5.9. We again fill the orbitals according to Hund's rule and the Pauli principle, beginning with the orbital that is lowest in energy.
Electrolysis is a process that splits hydrogen from water using an electric current. Electrolysis is commonly used to demonstrate chemical reactions and hydrogen production in high school science classes. On a large, commercial scale, the process may be referred to as power-to-gas, where power is electricity and hydrogen is gas.
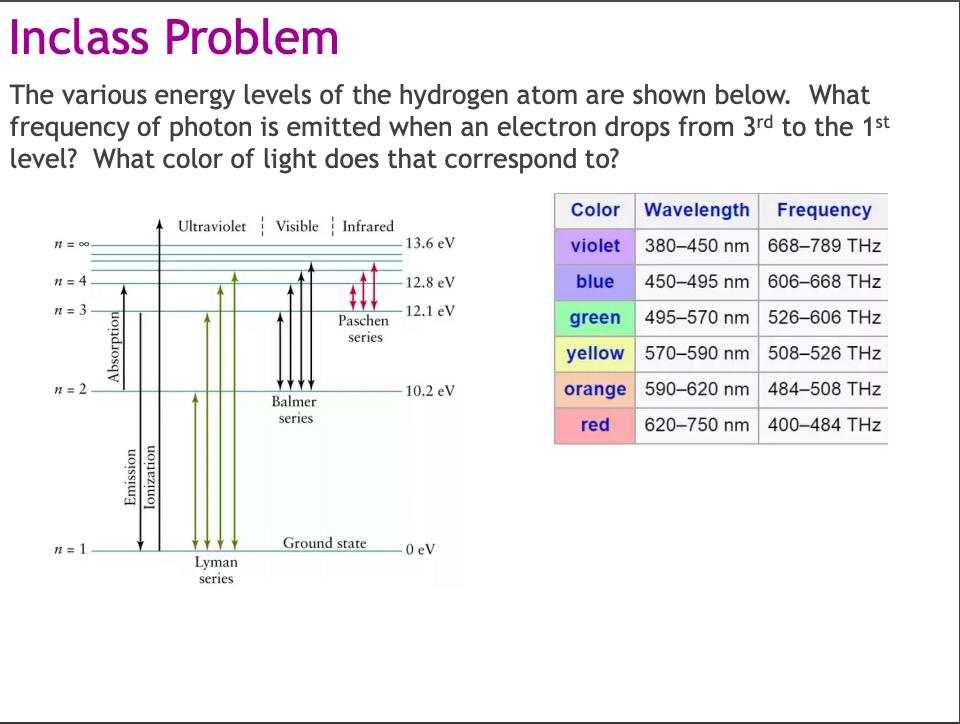
Each color of light has a particular energy and frequency. ... The image below shows an energy diagram showing possible transitions between the first seven energy levels in the hydrogen atom. Each ...
PH3 Lewis Structure, Molecular Geometry, and Hybridization. The compound Phosphorous Trihydride (PH3), also known as phosphine consists of phosphorus and hydrogen atoms. It is an inflammable and toxic gas without any color. Phosphine does not have any odor when it is pure, but most samples of the gas have the unpleasant odor of rotten garlic or ...
This can be studied with the help of the Valence Bond Theory (VBT) which says bonding takes place in such a manner that each molecule reaches a stable energy level with no strong repulsion, what-so-ever. The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals.
Previous studies have found that 4-VDPA is not a good sensitizer for Eu 3+ ions, mainly due to energy level mismatch between the triplet state energy level of 4-VDPA and the resonance level of Eu 3+ ions .The appearance of red fluorescence shows that 4-VDPA had undergone polymerization reaction to transform a DPA residue, which is considered as ...
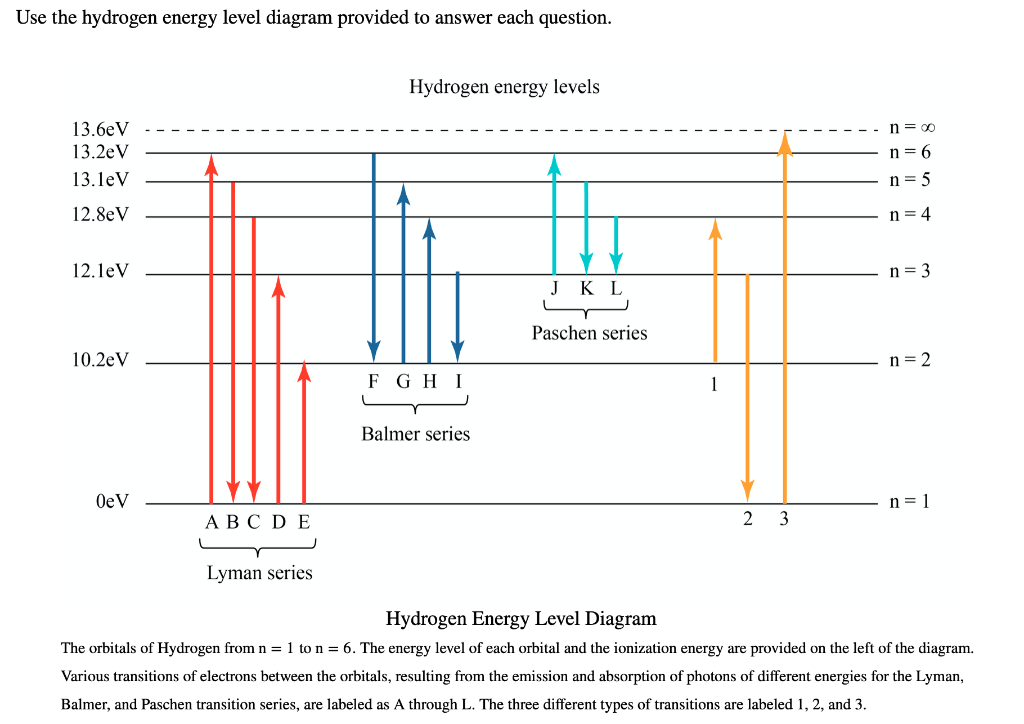
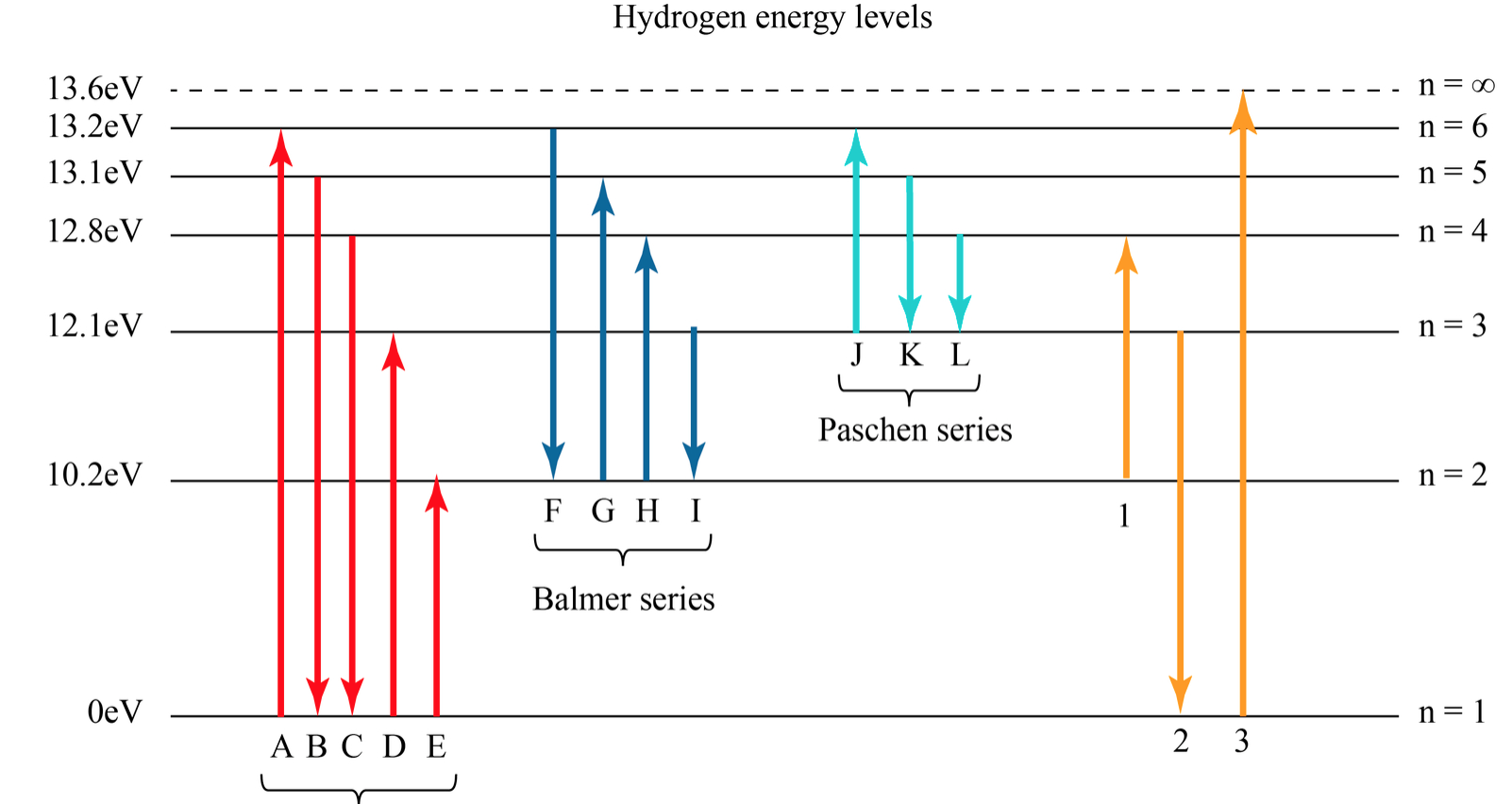
Next to it on the right is a diagram of the energy level that the electron occupies. Your job is to identify whether the atom makes a transition that involves the emission or absorption of a photon. Press the "next transitions" button to cause the hydrogen atom to undergo a transition to a higher - or maybe lower - energy state.
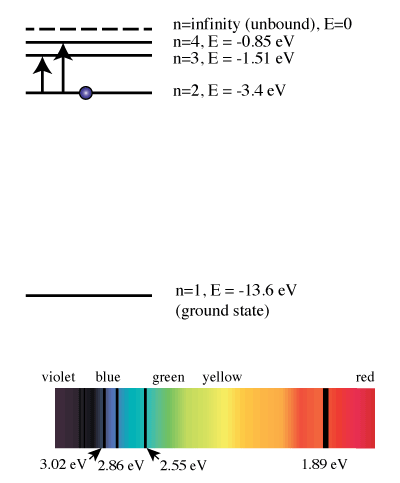
The emitted light's color and wavelength (λ) depend on the magnitude of the energy change (ΔΕ) due to the transition from one energy level to another. This is ...
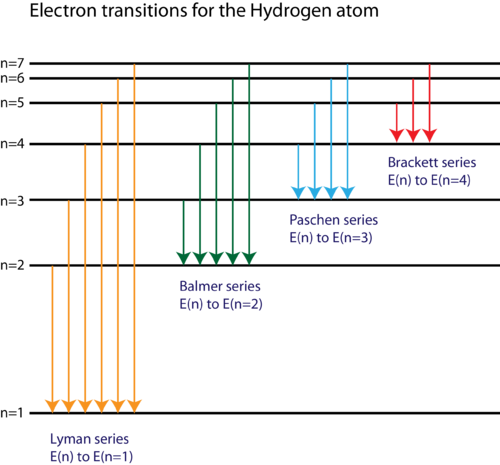
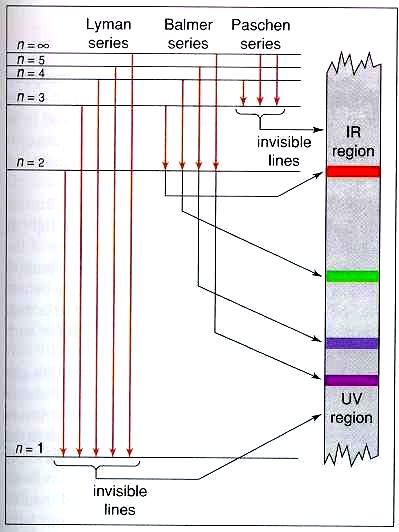
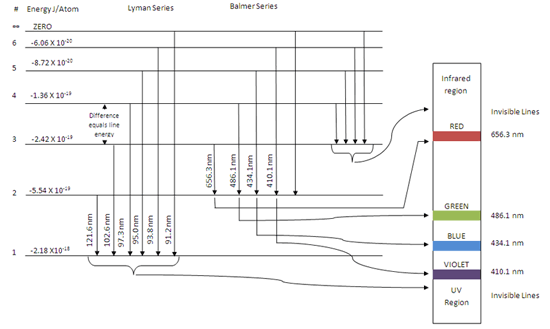
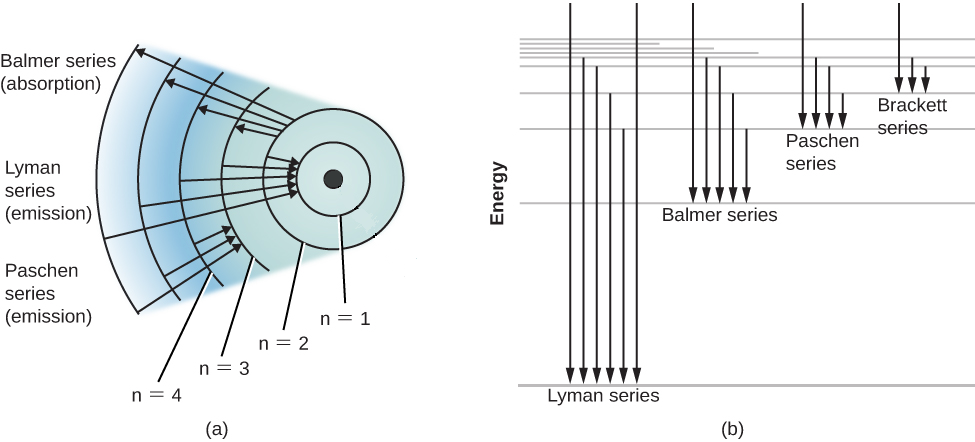
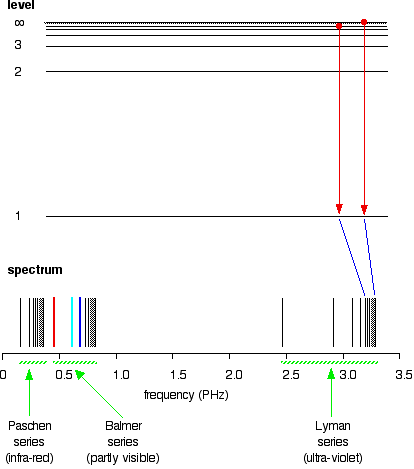
(1) When the electron jumps from energy level higher than n=1 ie. n=2,3,4,5,6 ….to n=1 energy level, the group of lines produced is called lyman series. These lines lie in the ultraviolet region. (2) The group of lines produced when the electron jumps from 3rd, 4th ,5th or any higher energy level to 2nd energy level, is called Balmer series.
The diagram to the right attempts to explain what transitions in a hydrogen atom cause which of the light bands. Since different colors are seen in the ...
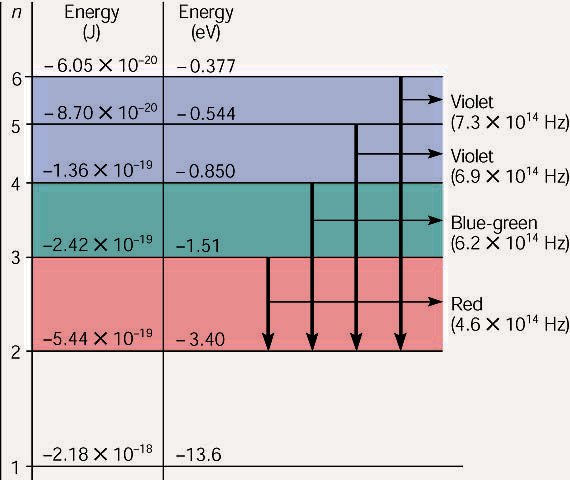
This is explained in the Bohr model by the realization that the electron orbits are not equally spaced. The electron energy level diagram for the hydrogen atom. He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level (n = 2).
Aug 21, 2020 — This page introduces the atomic hydrogen emission spectrum, ... The next few diagrams are in two parts, with the energy levels at the top ...
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...
These narrow bands have the characteristic wavelengths and colors shown in the table below. ... The energy levels of the hydrogen atom are quantized.Emission Spectrum of Hydrogen · Bohr Model of the Atom · Wave-Particle Duality
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...
You are already familiar with the fact that a prism can be used to disperse (separate) the colors of white light (like light from the sun or a light bulb) into ...5 pages
The electron energy level diagram for the hydrogen atom. He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level \(\left( n=2 \right)\). This is called the Balmer series. Transitions ending in the ground state \(\left( n=1 \right)\) are called the Lyman series, but the ...
List the electron transition lines according to energy levels. Explain the presence of emission ... The electron energy level diagram for the hydrogen atom.
An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons. These are obtained by substituting all possible values of \(n\) into Equation \(\ref{17.1}\).
Hydrogen Spectrum · Violet (380-435nm) · Blue(435-500 nm) · Cyan (500-520 nm) · Green (520-565 nm) · Yellow (565- 590 nm) · Orange (590-625 nm) · Red (625-740 nm) ...













0 Response to "40 hydrogen energy level diagram colors"
Post a Comment