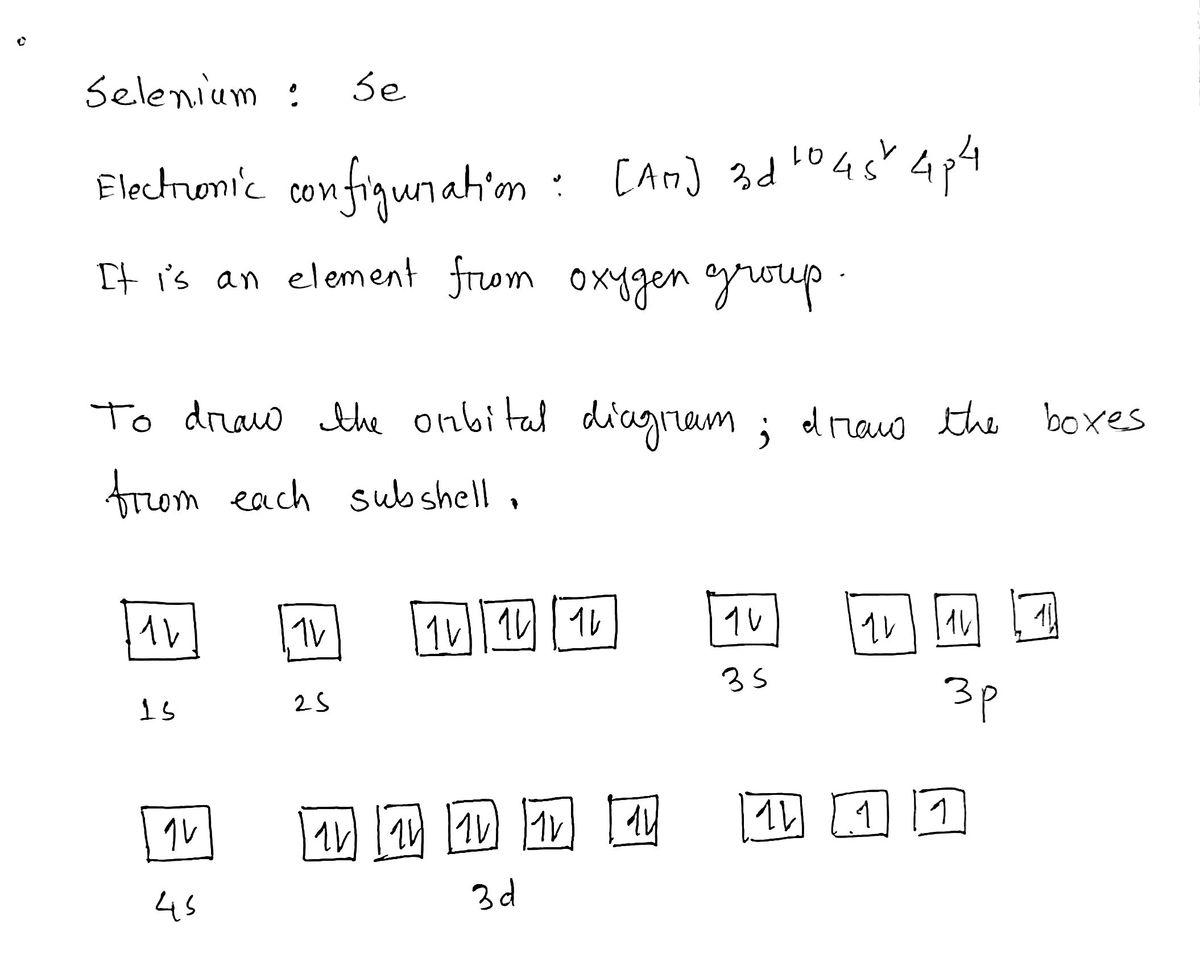
40 orbital diagram for se
01/10/2013 · Orbital neoplasms in adults may be categorized on the basis of location and histologic type. Imaging features of these lesions often reflect their tissue composition. Cavernous malformations (also known as cavernous hemangiomas), although not true neoplasms, are the most common benign adult orbital tumor. They typically appear as a well-circumscribed, ovoid …
An orbital diagram naturally leads to the writing of an electron configuration. ... Se-. [ar] 3d. 7. An ion of an isotope has a 2+ charge, an atomic mass of ...4 pages
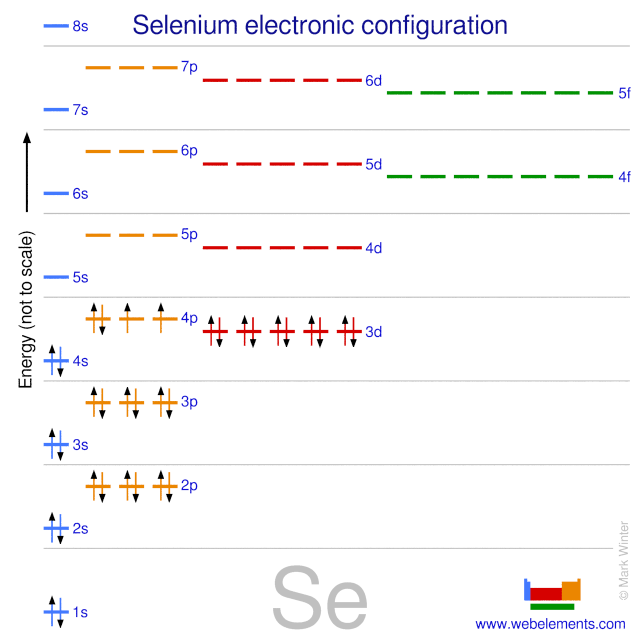
The ground state electron configuration of ground state gaseous neutral selenium is [Ar].3d10.4s2.4p4 and the term symbol is 3P2.

Orbital diagram for se
Selenium (Se) ; Oxidation States, +4,-2,+6 ; Electrons Per Shell, 2 8 18 6 ; Electron Configuration, [Ar] 4s2 3d10 4p ; Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. ↿ ...Atomic Number: 34Group: 16 (oxygen family) VIB (IUPAC), VIA ...Atomic Weight: 78.96 Isotopes
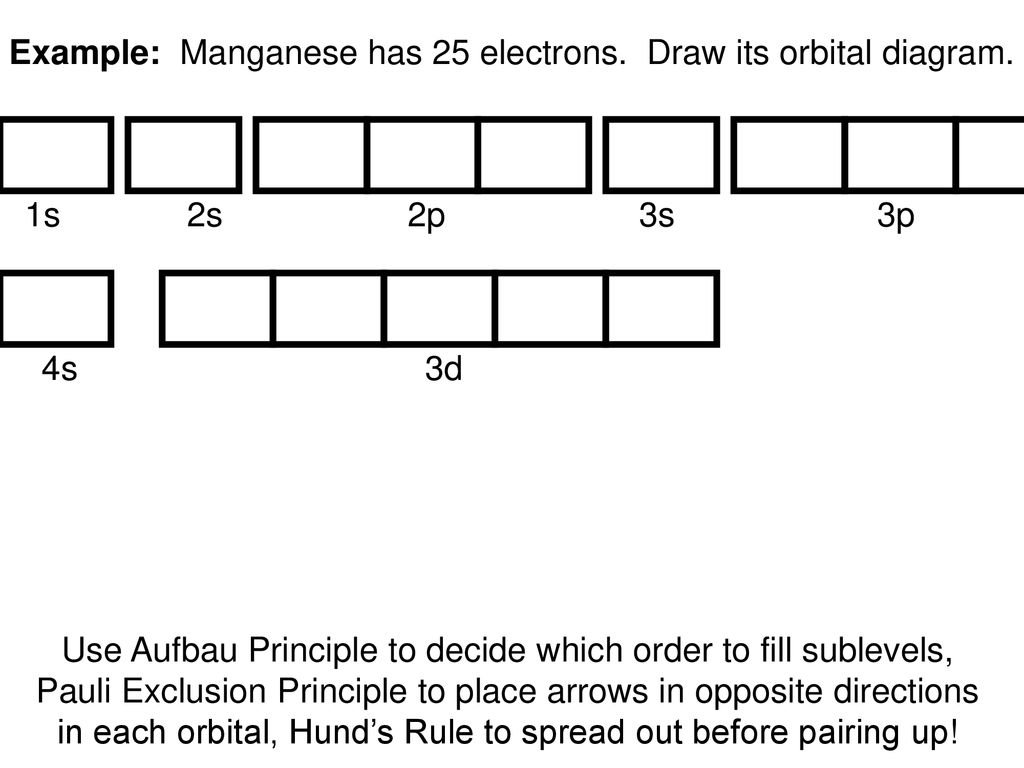
The third electron goes into the next orbital in the energy diagram, the 2s orbital. Li (Z = 3): 1s 2 2s 1. The fourth electron fills this orbital. Be (Z = 4): 1s 2 2s 2. After the 1s and 2s orbitals have been filled, the next lowest energy orbitals are the three 2p orbitals. The fifth electron therefore goes into one of these orbitals. B (Z = 5): 1s 2 2s 2 2p 1. When the time comes to add a ...
20/02/2021 · Orbital Diagram For Carbon (C) | Carbon Electron Configuration. February 20, 2021 by Sneha Leave a Comment. Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept. But if you are new here and looking for the information related to the carbon element and its electronic …
Orbital diagram for se.
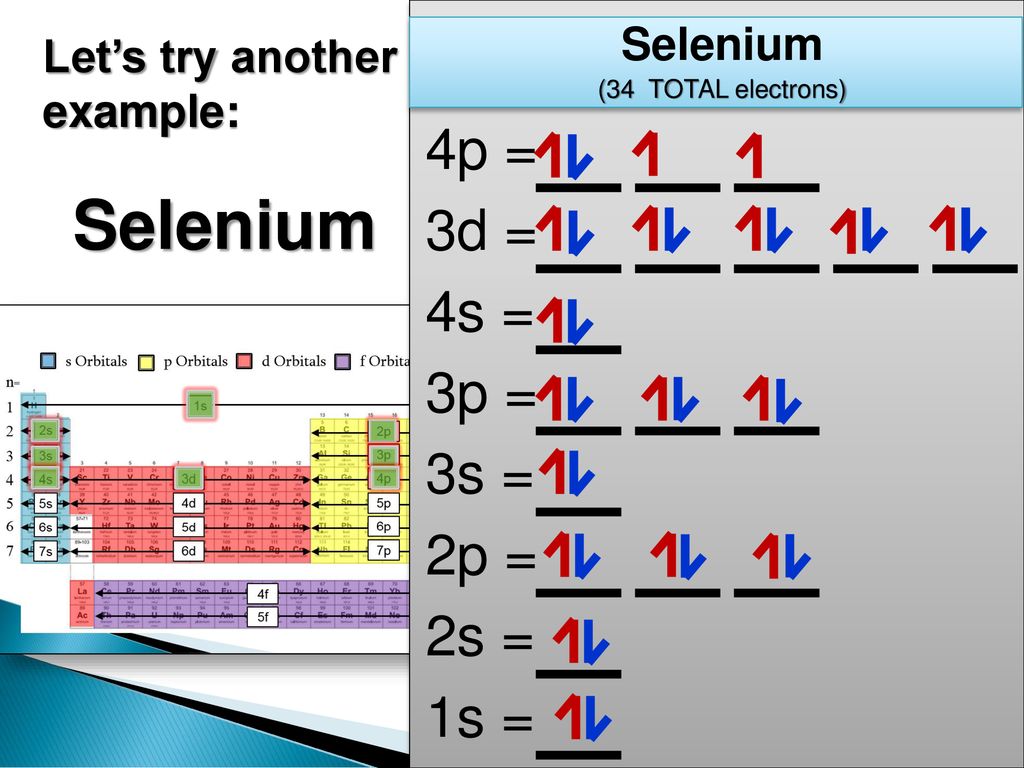
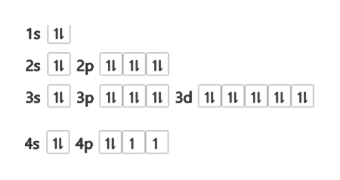
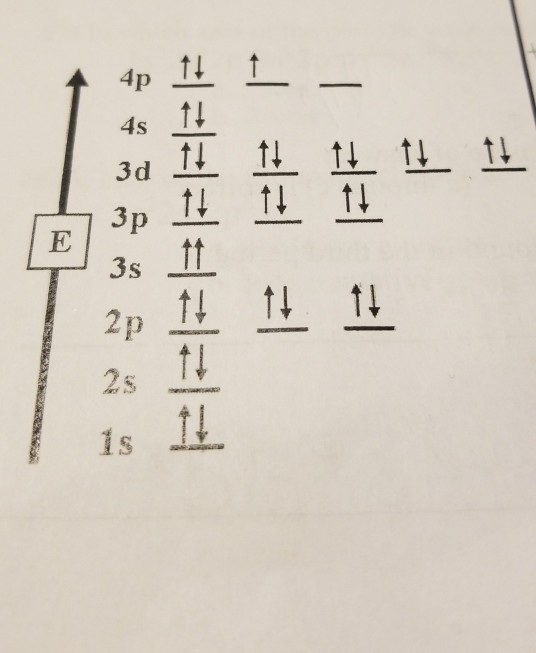
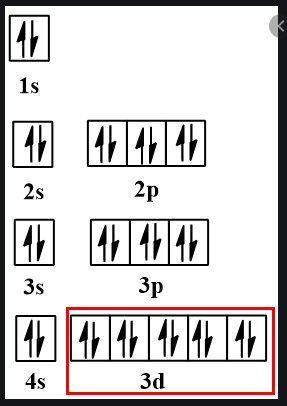
28 Jan 2021 — Selenium consists of 34 electrons distribution in its 4 orbits. So electronic configuration of selenium define as: 1s22s22p63s23p63d ...
Electronic configuration of the Selenium atom. Valence electrons. Orbital diagram.
Match each element (Sr, Se, Cl, Kr, Rb, Si) with its full ground-state electron configuration. (a) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 1 (b) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 (c ...
1 answerSelenium has 34 elements. Its condensed electronic configuration is represented as {eq}\left[ {{\rm{Ar}}}...
01/11/2021 · Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43: Orbital diagram of Technetium …
This website makes extensive use of JavaScript. The top menus will not function without it and most tools will also not work. If you do not know how to enable JavaScript in your web browser, you should be able find instructions by searching the web for "enable javascript in …
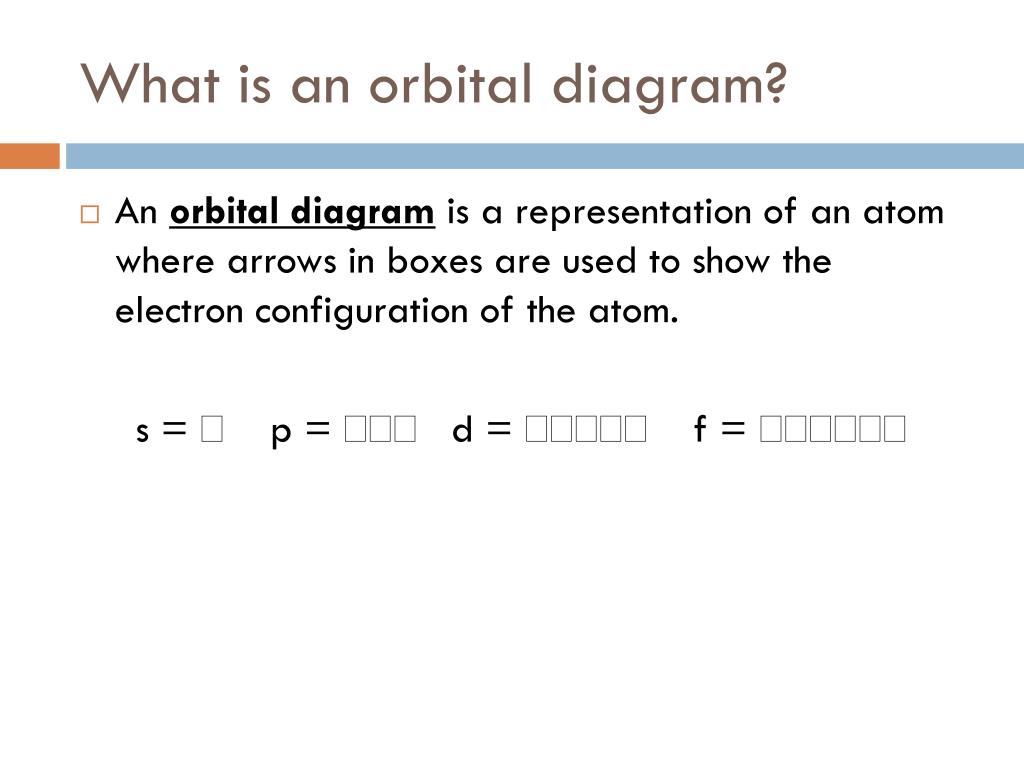
15/02/2021 · When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it. If you are new to such a subject and looking for periodic tables and their other information, then you can surely refer to our article. The good …
30 May 2020 — The electron configuration of an atom indicates the number of valence electrons. Valence electrons determine the unique chemistry of each ...
In this type of diagram, the molecular orbitals are represented by horizontal lines; the higher a line the higher the energy of the orbital, and degenerate orbitals are placed on the same level with a space between them. Then, the electrons to be placed in the molecular orbitals are slotted in one by one, keeping in mind the Pauli exclusion principle and Hund's rule of maximum …
3 Jul 2021 — The Electron Configuration of the Selenium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁴. You can also show the electronic configuration of the elements ...
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics.It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic …
The reason for this seeming anomaly is that the energy level of the s orbital in electron shell 4n (designated as the 4s orbital) is actually slightly lower than the energy level of the d orbitals in electron shell 3n, and we have already seen that electrons will (usually) occupy the orbitals with the lowest energy levels first (just to make things more complicated, the energy level of the 4s ...
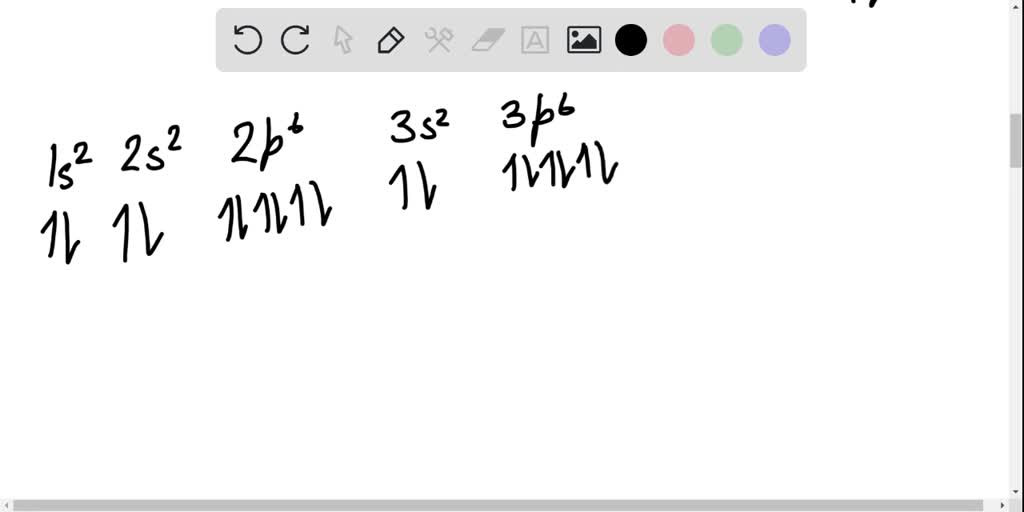
Write the electron configuration full or condensed for the element selenium and based on the electron orbital diagram indicate how many unpaired valence electrons there are in this element a 44583

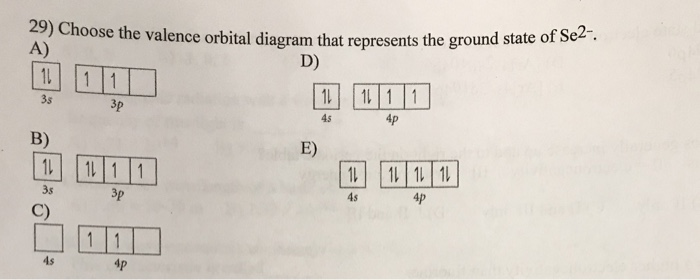





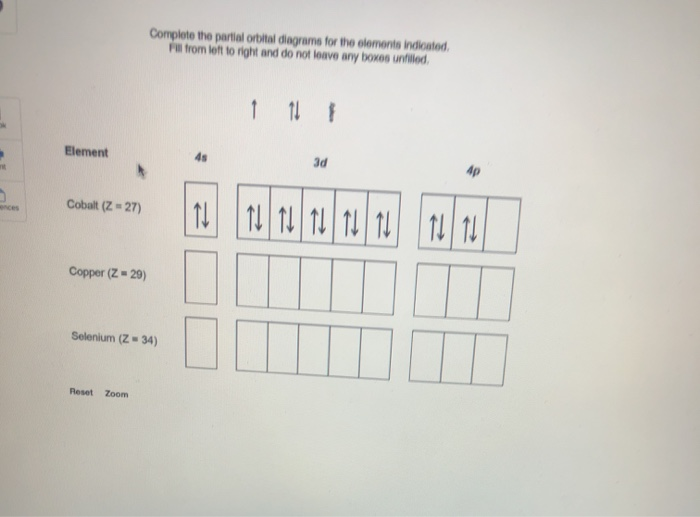
![6] (a) Write an orbital diagram for the ground state of Magnesium ...](https://img.homeworklib.com/images/b4358686-1dc6-4c4c-9ed3-6dd71a4b0aa1.png?x-oss-process=image/resize,w_560)
















0 Response to "40 orbital diagram for se"
Post a Comment