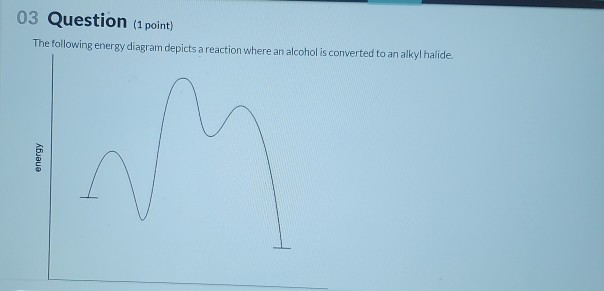
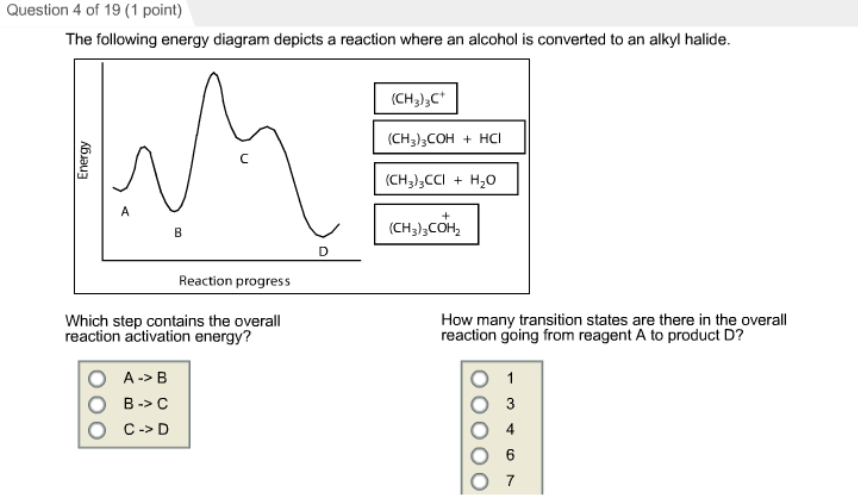
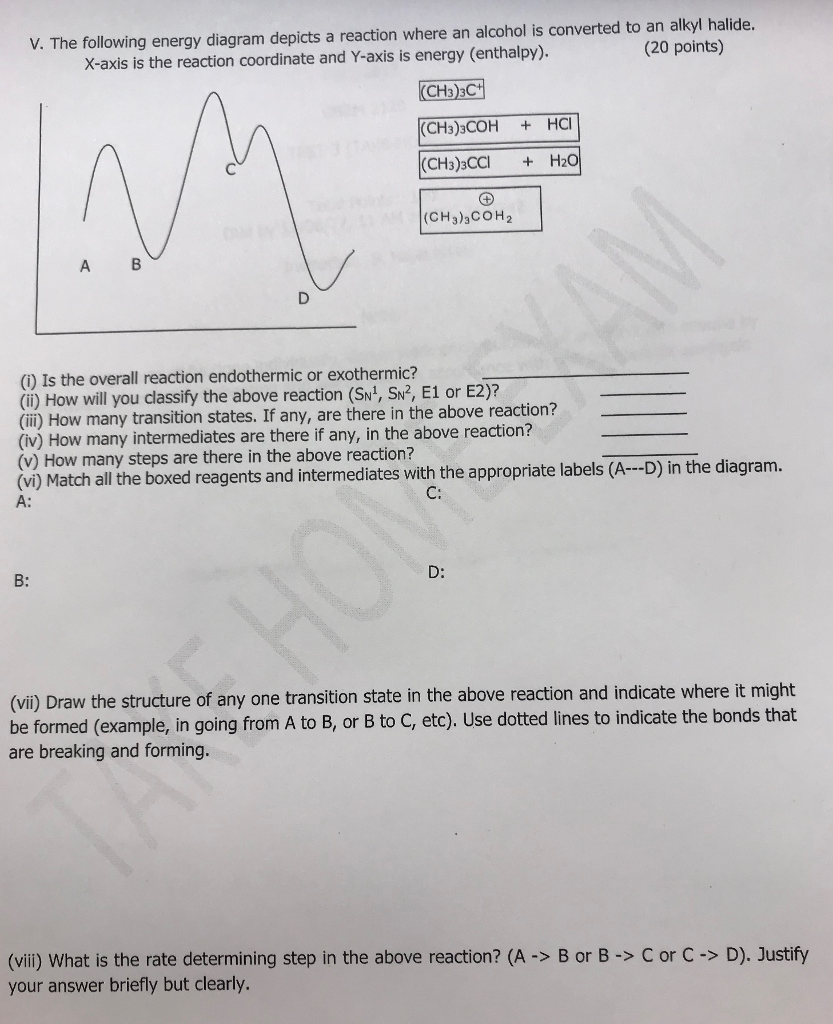
35 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
The following reactions are all examples of decarboxylation (loss of CO 2). In the first, bromine replaces the carboxyl group, so both the carboxyl carbon atom and the remaining organic moiety are oxidized. Silver salts have also been used to initiate this transformation, which is known as the Hunsdiecker reaction. The second reaction is an ... These reactions are endothermic and can be represented by an energy-level diagrams like Figure 7.12 (lower). Exothermic and endothermic reactions can be thought of as having energy as either a "product" of the reaction or a "reactant", respectively. Exothermic reactions release energy, so energy is a product. Endothermic reactions ...
Alkyl halide or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic precursors such as alkanes, alkenes, or alcohols and carboxylic acids. Generally, alkyl halides contain hydrogen atoms attached to the sp ...
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
Answer: C Section: 7.2. Circle all atoms that are coplanar in the molecule below. Section: 7.2. Draw the line energy orbital diagram for the outer shell of an sp2 hybridized carbon atom and explain how a carbon/carbon double is formed. If we follow Hund's rule and fill each orbital with one electron and then promote one to the lone atomic p ... Solution for energy The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. reaction progress Part 1 See Periodio ... 2.1 Amino Acid Structure and Properties. Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Proteins may be structural, regulatory, contractile, or protective; they may serve in transport, storage, or membranes; or they may be toxins or enzymes.
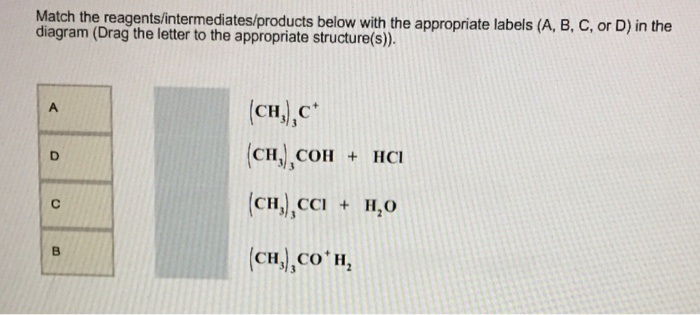
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. The alkyl hydrogen sulfate can be converted to an alcohol by boiling in water. This proceeds usually by S N 1 substitution where water is the nucleophile and bisulfate is the leaving group. The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene. (Markovnikov orientation). The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. Match the reagents/intermediates/products below with the appropriate labels (A, B, C, or D) in the diagram (Drag the letter to the appropriate structure(s)). SN2 reactions are a form of nucleophilic substitution, ... Ethers can be converted into an alkyl halide and a alcohol through protonation by a strong acid, ... Dec 11, 2019 — The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. Match the reagents/intermediates/products ...
The reactions of acid anhydrides are slower than the corresponding reactions with acyl chlorides, and you usually need to warm the mixture. Taking ethanol reacting with ethanoic anhydride as a typical reaction involving an alcohol: There is a slow reaction at room temperature (or faster on warming). 5. Question 5 - The Energy Diagram of SN2 reaction: Draw an energy diagram for the following S N 2 reaction. Label the axes, the Ea, the ΔH° and the transition state of the reaction.Assume the reaction is exothermic and ΔH° = -75 kJ/mol and Ea = 50 kJ/mol. Draw the structure of reactants and products on the diagram.You can put the reactants at any energy level and then draw the rest as ... Minnesota State University Moorhead The Grignard reaction is an organic reaction used to produce a variety of products through the reaction of an organomagnesium compound, also known as an electrophilic "Grignard reagent," followed by an acidic reaction. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism.
1-Bromopropane is a colorless liquid. Commercial 1-bromopropane includes not only 1-bromopropane, but also additives that improve its performance in the desired application and stabilizers to inhibit decomposition.1-Bromopropane was originally used in the production of pesticides, flavors and fragrances, pharmaceuticals, and other chemicals. a) In a substitution reaction, the halogen X is replaced by a nucleophile. c) Alkyl halides undergo elimination reactions with Bronsted-Lowry bases. b) The C-X sigma bond is broken and the C-Nu sigma bond is formed in an elimination reaction. d) Elimination reactions lead to the formation of a new pi bond. The C-X sigma bond is broken and the C ... Step 1: formation of an alkyl cation as an ion pair Step 2: attack of the alkyl cation on the aromatic ring Step 3: proton transfer regenerates the aromatic ring + R+ R H R H R H A resonance-stabilized cation + + + H R + Cl AlCl 3 R ++AlCl3 HCl RCl ClAl Cl Cl RCl Cl Cl Al Cl R+ AlCl 4-An ion pair containing a carbocation +-+ A molecular complex ... 16.4 ELECTROPHILIC AROMATIC SUBSTITUTION REACTIONS OF BENZENE 755 Step 2 Reaction of the benzene p electrons with the electrophile to form a carbocation inter- mediate. (Notice that either of the oxygens can accept the electron pair.) Step 3 Loss of a proton from the carbocation to give a new aromatic compound. Nitration is the usual way that nitro groups are introduced into aromatic rings.
Reaction Coordinate Diagram of an S N 2 Reaction. The reaction of hydroxide ion with chloromethane occurs in a single step. The activation energy, E a, reflects the stability of the transition state, which depends upon the structure of the substrate, the nucleophile, and the leaving group.
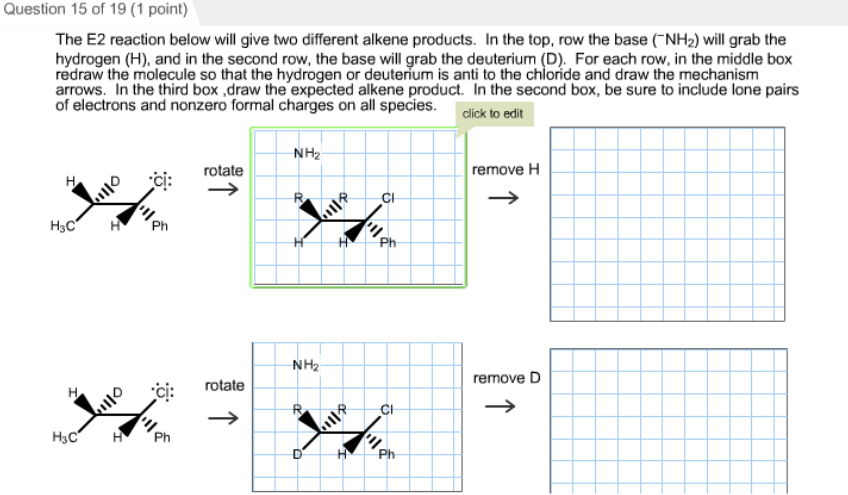
The S N 2 Reaction Notes: In the SN2 reaction, the nucleophile attacks from the most δ+ region: behind the leaving group. This is called a back-side attack. This back-side attack causes an inversion (study the previous slide): after the leaving group leaves, the other substituents shift to make room for the newly-bonded nucleophile, changing the stereochemistry of the molecule.
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond, commonly called a carbonyl group. The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids and esters) described in this section.
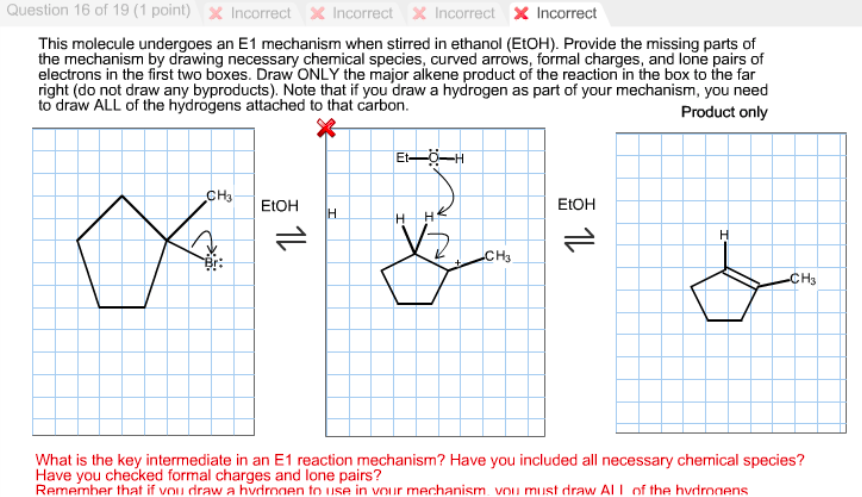
8.5. Elimination reactions. Alkyl halides undergo elimination via two common mechanisms, known as E2 and E1, which show some similarities to S N 2 and S N 1, respectively. In E2, elimination shows a second order rate law, and occurs in a single concerted step (proton abstraction at C α occurring at the same time as Cβ-X bond cleavage).
vibrate corresponding to discrete energy levels. These resonant frequencies are determined by the shape of the molecular potential energy surfaces, the masses of the atoms and, by the associated vibronic coupling. In order for a vibrational mode in a molecule to be IR active, it must be associated with changes in the permanent dipole.
higher than the energy of the reactants; a reaction in which heat is absorbed. Transition state An unstable species of maximum energy formed during the course of a reaction; a maximum on an energy diagram. FIGURE 5.1 An energy diagram for a one-step reaction between C and AJB. The dashed lines in the transition state indicate that the new CJA
Sep 12, 2020 — There are two mechanistic models for how an alkyl halide can undergo ... A potential energy diagram for this reaction shows the transition ...Missing: depicts | Must include: depicts
In the presence of a strong acid, an alcohol can be dehydrated to form an alkene. The acid used in this experiment is 85% phosphoric acid and the alcohol is cyclohexanol. The phosphoric acid is a catalyst and as such increases the rate of reaction but does not affect the overall stoichiometry. It can be seen from the balanced reaction
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. (CH3)3C (CH3) COH HCI (CH3)3 CCI H20 (CH3) COH Reaction progress O exothermic is the overall reaction exothermic or endothermic? O endothermic ; Question: The following energy diagram depicts a reaction where an alcohol is converted to an alkyl ...
The following energy diagram represents the alcohol is converted to an alkyl halide: [(CH3)ąc* Energy (CH3),COH + HCI (CH3),CCI + H2O | (CH₃CH₂ Reaction ...1 answer · 0 votes: Le relazcos (CH3 cars to (CH3)₂ COH the (alcohol) H2O + (CH 3 ) z c-cl alkyl chloride < (CH3)₂e@ theo horous the 2 OH2 Ha 2 C-ce @ to Realtim progress →
Reaction coordinate Free energy ‡ ΔG° ΔG Transition state CH 2 CH 2CH 2CH 3 Cl I + Cl− I Cl δ− δ− 6.5 I − (CH 3) 3 CBr I + 3 3 Br − 6.6 (a) We know that when a secondary alkyl halide reacts with hydroxide ion by substitution, the reaction occurs with inversion of configuration because the reaction is S N2. If we
Functional Groups with Carbon Singly Bondd l ided to an Electronegative Atom Alkyl halide:Alkyl halide: C bonded to halogen (CC bonded to halogen (C-X) Alcohol: C bonded O of a hydroxyl group (C OH) Ether: Two C's bonded to the same O (C O C) Amine: C bonded to N (C N) Thiol: C bonded to SH group (C SH) Sulfide: Two C's bonded to same S (C S C)
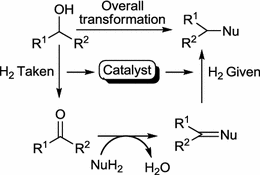
Wolff-Kishner Reduction. The reduction of aldehydes and ketones to alkanes. Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
It oxidises some of the alcohol to carbon dioxide and at the same time is reduced itself to sulphur dioxide. Both of these gases have to be removed from the alkene. It also reacts with the alcohol to produce a mass of carbon. There are other side reactions as well, but these aren't required by any current UK A level (or equivalent) syllabus.
As illustrated in the following diagram, a base induced intramolecular substitution reaction forms a three-membered cyclic ether called an epoxide. Both the halohydrin formation and halide displacement reactions are stereospecific, so stereoisomerism in the alkene will be reflected in the epoxide product ( i.e. trans-2-butene forms a trans ...
Academia.edu is a platform for academics to share research papers.
2.1 Amino Acid Structure and Properties. Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Proteins may be structural, regulatory, contractile, or protective; they may serve in transport, storage, or membranes; or they may be toxins or enzymes.
Solution for energy The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. reaction progress Part 1 See Periodio ...
Answer: C Section: 7.2. Circle all atoms that are coplanar in the molecule below. Section: 7.2. Draw the line energy orbital diagram for the outer shell of an sp2 hybridized carbon atom and explain how a carbon/carbon double is formed. If we follow Hund's rule and fill each orbital with one electron and then promote one to the lone atomic p ...























0 Response to "35 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide"
Post a Comment