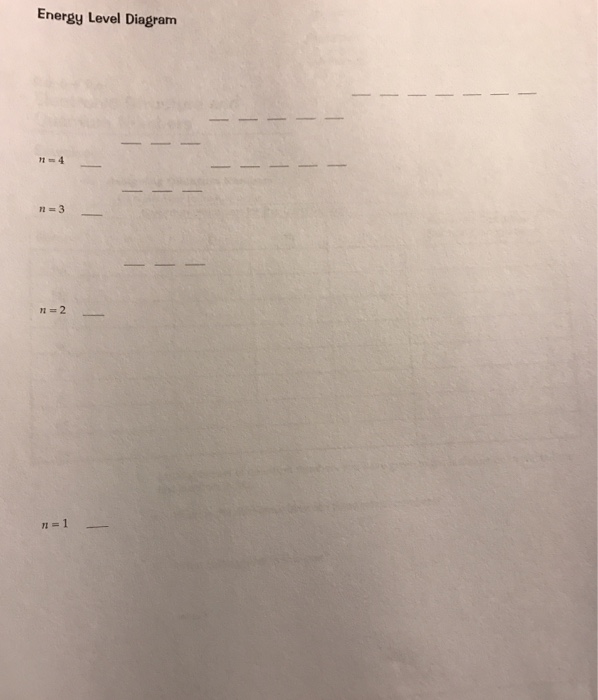
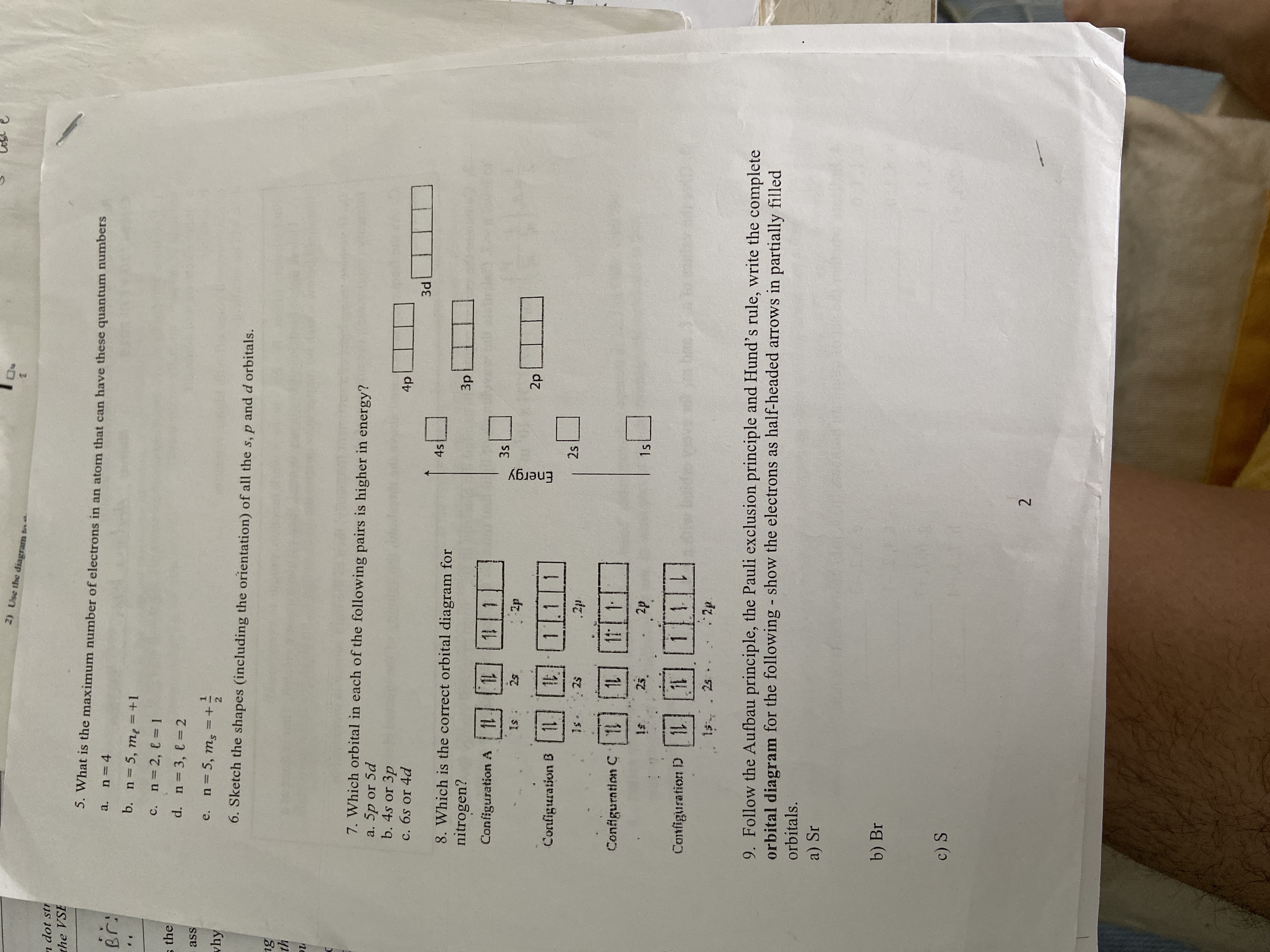
36 use the orbital diagram for nitrogen to write quantum numbers for the 3rd electron of the n atom.
December 8, 2019 - The orbital diagram is a type of diagram which shows the distribution of electrons in the orbitals of an atom and indicates the spin of those electrons. It is a type of notation which shows which orbitals are filled and which are partially filled. Here, we use arrows to represent electrons. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. 606 Pages. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition
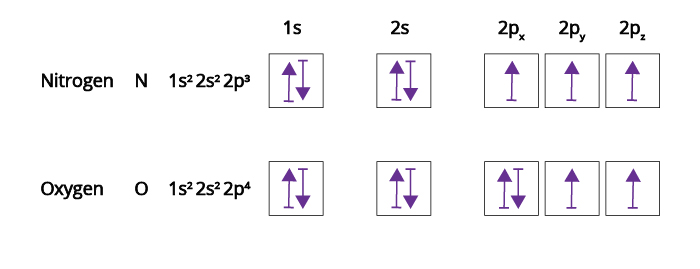
with 3 unpaired electrons. The electron configuration of nitrogen is therefore 1 s 22 s 22 p 3. At oxygen, v Z = 8 and also eight electrons, we have actually no choice. One electron must be combine with another in one of the 2 p orbitals, which provides us two unpaired electrons and a 1 s 22 s 22 p 4 electron configuration.

Use the orbital diagram for nitrogen to write quantum numbers for the 3rd electron of the n atom.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. OK I am having major fundamental issues picturing carrier behaviour in p-n junctions. I have 2 good examples of where my logic doesn't make sense: 1) In a p-n junction with a small forward bias with less than the band gap energy, some majority carriers are injected in their respective side, and the carrier diffuses across to recombine when it is the minority carrier. Now if i track this path along an Energy vs. distance axis, at some point I will find an energy change corresponding to the band ... I thought nitrogen is N2, and nitride is N. This took out some points in previous exams so I been very careful about it. After looking it up, it seems nitrogen is N2, nitrogen ATOMS is just N?
Use the orbital diagram for nitrogen to write quantum numbers for the 3rd electron of the n atom.. Boron (B) is atomic no. 5, and has an electron configuration of 1s2 2s2 2p1.Each of the 5 electrons has a set of quantum numbers. If you are referring to the last electron, i.e. the 2p1 electron, then they would be: n =2 l = 1 ml = -1 s = -1/2 Whether to reference us in your work or not is a personal decision. If it is an academic paper, you have to ensure it is permitted by your institution. We do not ask clients to reference us in the papers we write for them. When we write papers for you, we transfer all the ownership to you. Atoms of which element, indicated by letter on the periodic table, have the orbital-filling diagram shown below? A What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) moving at a velocity of 3.0 × 107 m/s (10% of the speed of light)? Photoexcitation of the Ir center leads to the transition of an electron from t 2g level to a molecular orbital which mainly has ligand character and therefore is not visible in XAS. The vacancy at e g states can be well observed in XAS and therefore, the photoexcitation leads to the appearance of positive peak A 1 of the transient XAS spectrum ...
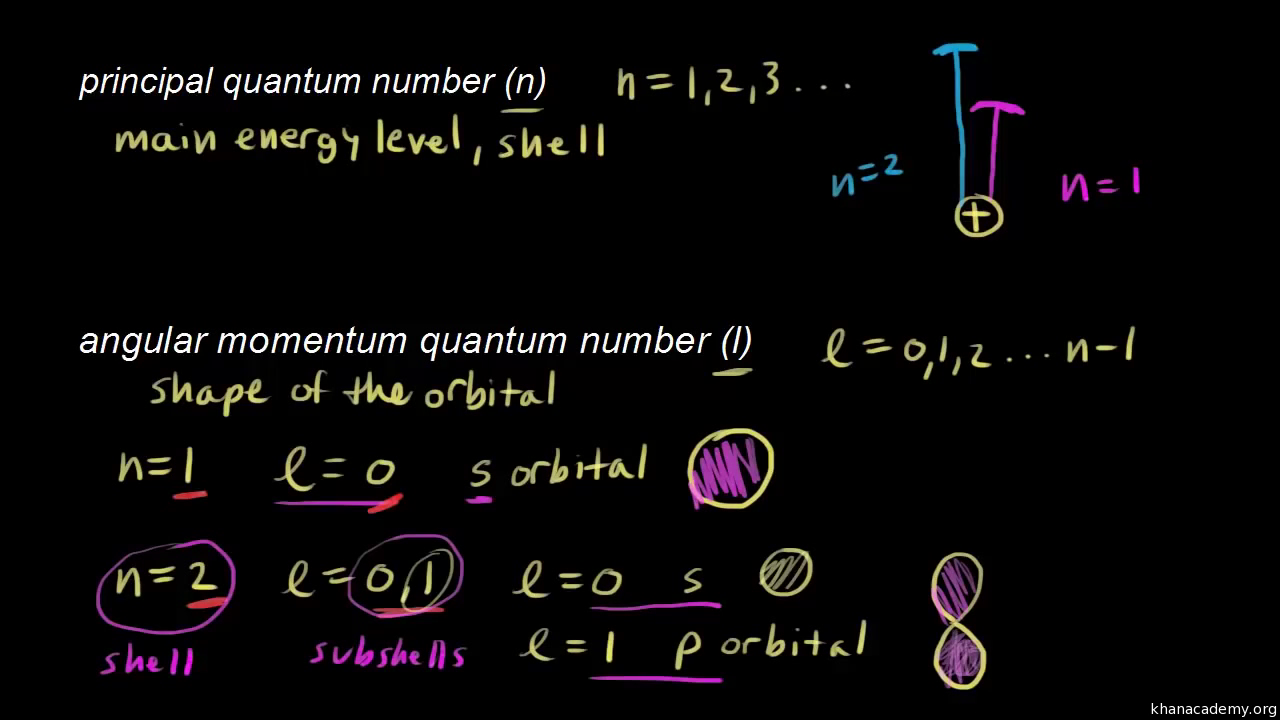
*How* exactly do things that seem *totally* (1) abstract and (2) exotic and (3) inconsequential and (4) non-phenomenological and (5) otherworldly and (6) arbitrary, such as how many nucleons/electrons are present in an atom, possibly determine elements' properties in the real world? I look at the Periodic Table. I look at the atomic weights. It all seems like an entirely different world. And yet somehow it gives rise to the differences between everything. I mean, how can it all be the same... Since the sublevel is 3p, we can see that the principal quantum number (n) = 3. The angular quantum number (l) that corresponds with sublevel p is 1. When l = 1, possible values for quantum number m l are -1, 0 and +1. Similarly one may ask, what are NLM quantum numbers? 2 days ago - Selenium (Se) atom electron configuration (Bohr model) K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n 2. For example, n = 1 for K orbit. The electron holding capacity of K orbit is 2n 2 ... And Pauli’s exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. To write the orbital diagram of krypton(Kr), you have to do the electron configuration of krypton. Which has been discussed in detail above.
Oct 11, 2021 · Draw the energy level diagram for c, H, o, N. P. and s with electrons filled properly in the orbitals; Write down the electron configuration and draw Lewis dot structure for each atom. When an ... From my understanding, the electron configuration for La is: [Xe]5d1 6s2 From there, I think that the answer is: N=6, l= 0, ml=0, spin=-1/2 Is that correct? Here's what I got. Boron, "B", is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which ... I’m obviously talking about this in the Bohr model sense.
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in ...
Section 1.6 - 3 In polyelectronic atoms the motions and spins of the individual electrons are correlated due to their electrostatic and spin-spin interactions. Consequences: a) Only electronic configurations that do not violate the Pauli Principle are allowed. b) The different arrangements ...
Rubidium (Rb) atom electron configuration (Bohr model) K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n 2. For example, n = 1 for K orbit. The electron holding capacity of K orbit is 2n 2 ...
Drop all the files you want your writer to use in processing your order. If you forget to attach the files when filling the order form, you can upload them by clicking on the “files” button on your personal order page. The files should be uploaded as soon as possible to give the writer time to review and use them in processing your order.
The principal quantum number (n): ... How many electrons are unpaired in the orbitals of nitrogen? 3 "When filling orbitals of equal energy, electrons fill them singly first with parallel spins." This is known as: Hund's rule. An accepted abbreviation format is to write an electron configuration ...
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next ...
March 12, 2020 - Atomic size increases as you move down a column because the outermost electrons occupy orbitals with a higher principal quantum number that are therefore larger, resulting in a larger atom. C.) Al atoms are larger than N atoms because as you trace the path between N and Al on the periodic table, ...
This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms) from an element or valence. This video provides 3 example practice problems showing you how to write the quantum numbers using the electron configuration.
It's fairly self-explanatory - the further to the right a dot is, the more of a "leave" constituency it was in 2016. Colourings reflect the result of the 2017 GE, so no Tiggers in there. Quite a strong link between the two factors, which isn't a huge surprise. https://preview.redd.it/uwmep9pxckn21.png?width=2500&format=png&auto=webp&s=bce96c574a1abd7cdce7edba1c1fe3af992215c9
Click within the orbital to add electrons. In the same way, the orbital filling diagram for nitrogen will be: It’s not until we reach oxygen, where the electrons will start to double up, just because there’s no alternative: And that’s the basic idea behind orbital filling diagrams!
Consider the two electron arrangements for neutral atoms A and B. What is the difference between atom A and atom B? A - 1s22s22p63s1 B - 1s22s22p65s1 Atom B has lost some inner electrons. The outer electron of atom B has moved to a lower energy state. The outer electron of atom B has moved to a higher energy state.
2 days ago - And Pauli’s exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. To write the orbital diagram of bromine(Br), you have to do the electron configuration of bromine. Which has been discussed in detail above.
The electrons in multielectron atoms can be identified through four quantum numbers (principal, angular, magnetic, and spin) in the order.n 1 ... Three-electron atoms L 3 assumes, for each possible value of L 2, all the values in the interval L 2 C ` 3;L 2 C ` 3 1:::jL 2 ` 3j.
What would the orbital diagram for these molecules look like if they had this many electrons? ​ I'd guessed that phosphorus would have 3s(2), 3p(3), and 3d(5). The half-filled p and d orbitals would offer stability to explain why phosphorus can sometimes make 5 bonds. Alternatively I was considering 3s(2), 3p(6), and 4s(2). ​ I'd guessed that sulfur would have 3s(2), 3p(3), 3d(5), and 4s(2). Again, the half-filled p and d orbitals would offer stability. An electron from...
(1) n = 1, 2, 3, and so on. (2) ℓ = 0, 1, 2, . . . , n - 1 (3) m ℓ starts at negative ℓ, runs by whole numbers to zero and then goes to positive ℓ. (4) after the n, ℓ and m ℓ to be used have been determined, assign the m s value +½ to one electron, then assign the m s value of ...
Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
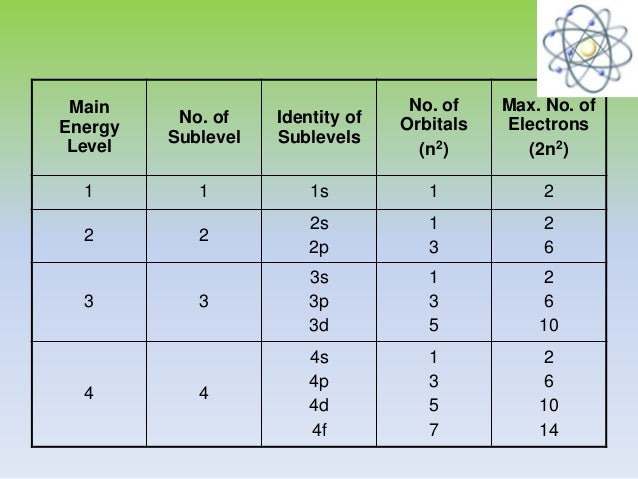
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, ...
I thought nitrogen is N2, and nitride is N. This took out some points in previous exams so I been very careful about it. After looking it up, it seems nitrogen is N2, nitrogen ATOMS is just N?
OK I am having major fundamental issues picturing carrier behaviour in p-n junctions. I have 2 good examples of where my logic doesn't make sense: 1) In a p-n junction with a small forward bias with less than the band gap energy, some majority carriers are injected in their respective side, and the carrier diffuses across to recombine when it is the minority carrier. Now if i track this path along an Energy vs. distance axis, at some point I will find an energy change corresponding to the band ...
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.

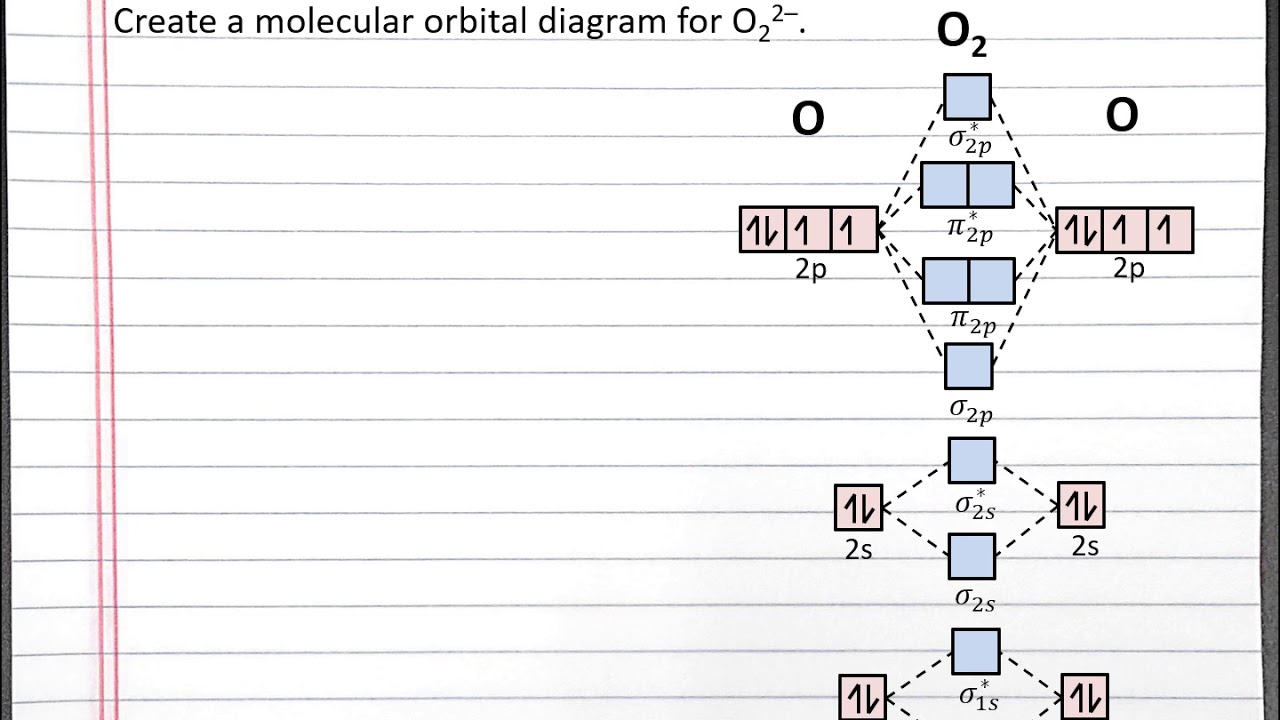



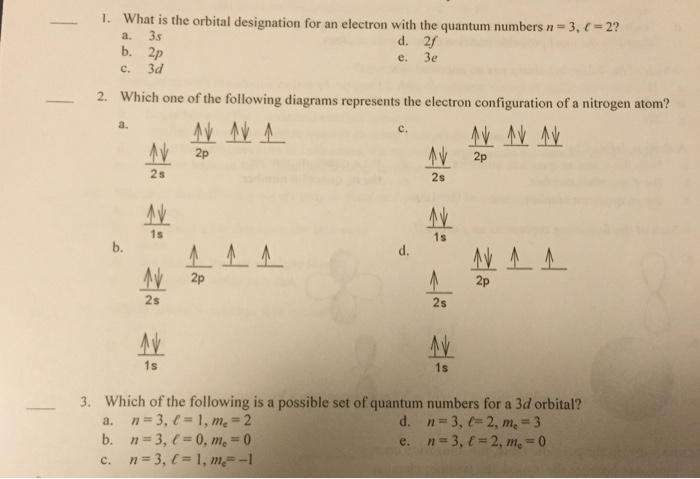





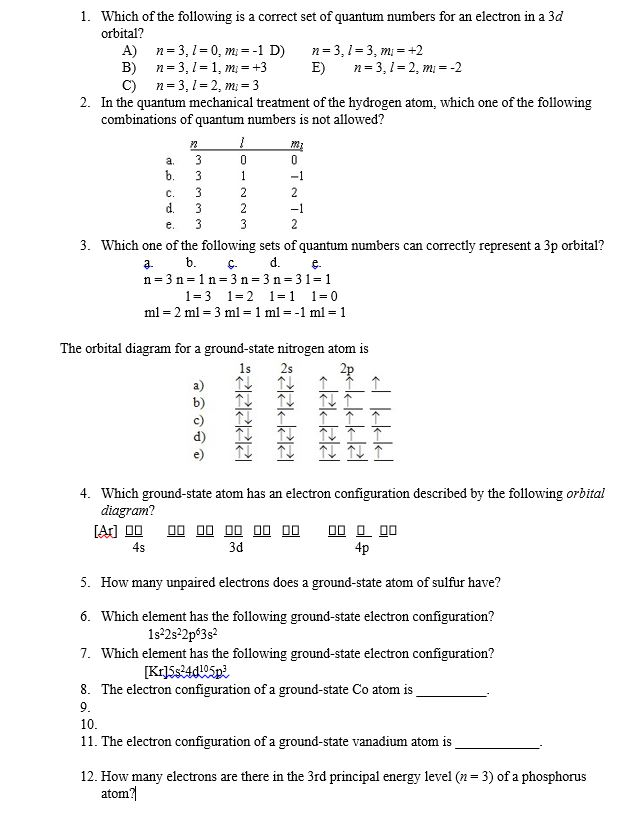



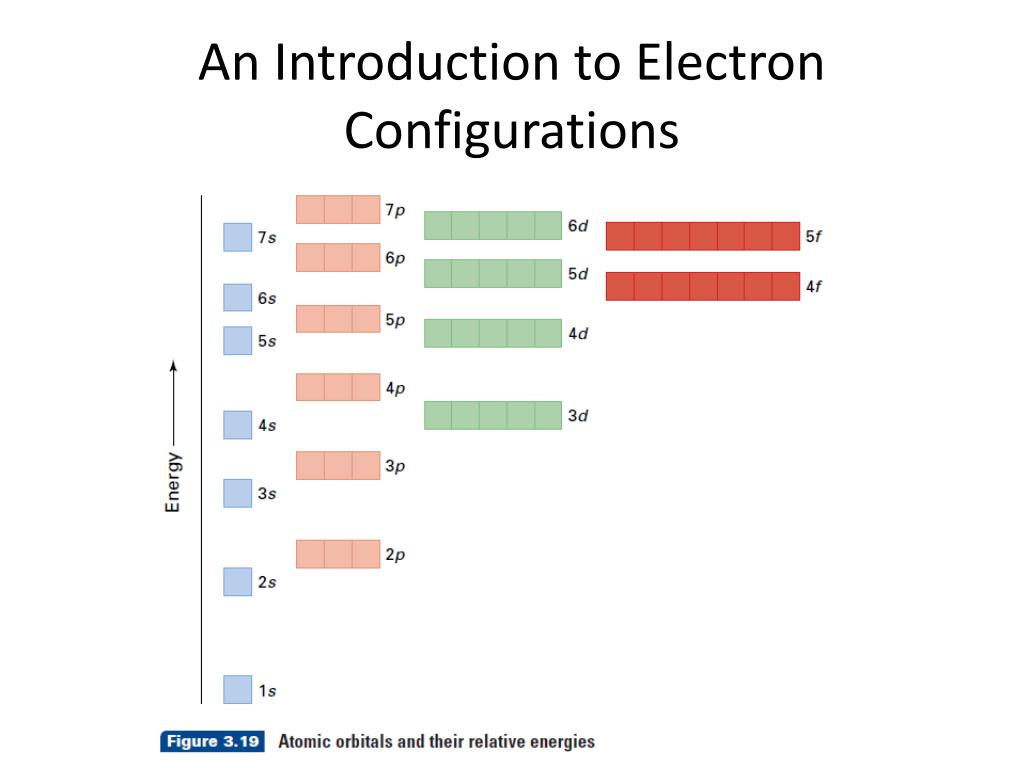









0 Response to "36 use the orbital diagram for nitrogen to write quantum numbers for the 3rd electron of the n atom."
Post a Comment