36 orbital diagram of carbon before sp3 hybridization
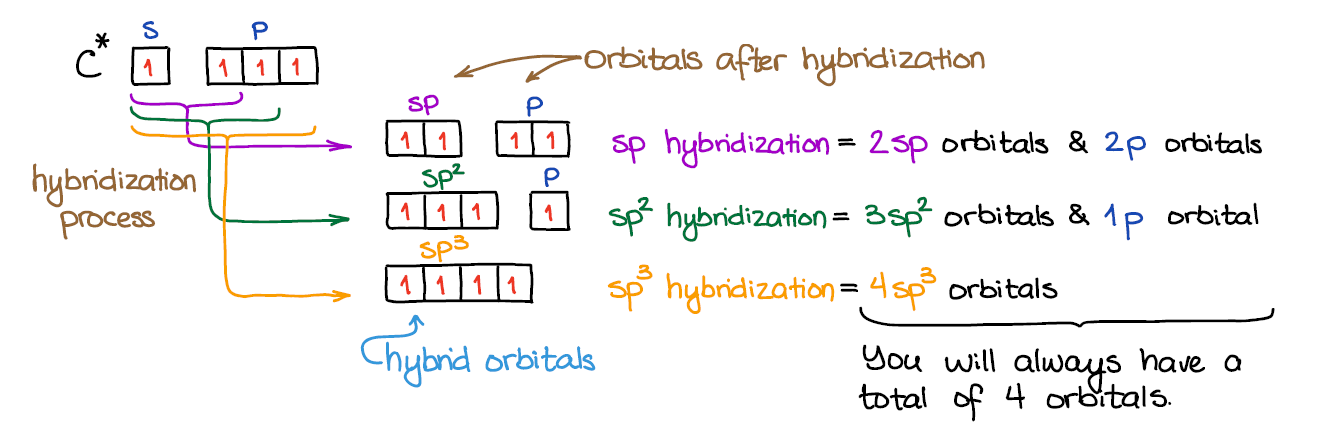
Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Use the buttons at the top of the tool to add orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more … sp3, sp2, and sp Hybridization in Organic Chemistry with In sp hybridization, the s orbital of the excited state carbon is mixed with only one out of the three 2p orbitals. It is called sp hybridization because two orbitals (one s and one p) are mixed: The ...
About Albr3 hybridization . With this result, the Br 3d 3/2 and Br 3d 5/2 binding energies of Br atoms in AlBr 3 are (0. I would like to ask if anyone knew the orbital box hybridization diagram for ClF3. mx. I want to try another solution. Use principles of atomic structure and/or chemical bonding to answer each of the following.
:max_bytes(150000):strip_icc()/hybrid-orbital-38d0c0f547ef46268127f68ce20714fa.jpg)
Orbital diagram of carbon before sp3 hybridization
Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. Example: Hybridization of graphite. sp3 Hybridization: When the carbon atom is bonded to four other atoms. Question: write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. This problem has been solved! See the answer ... Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Q. Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics.
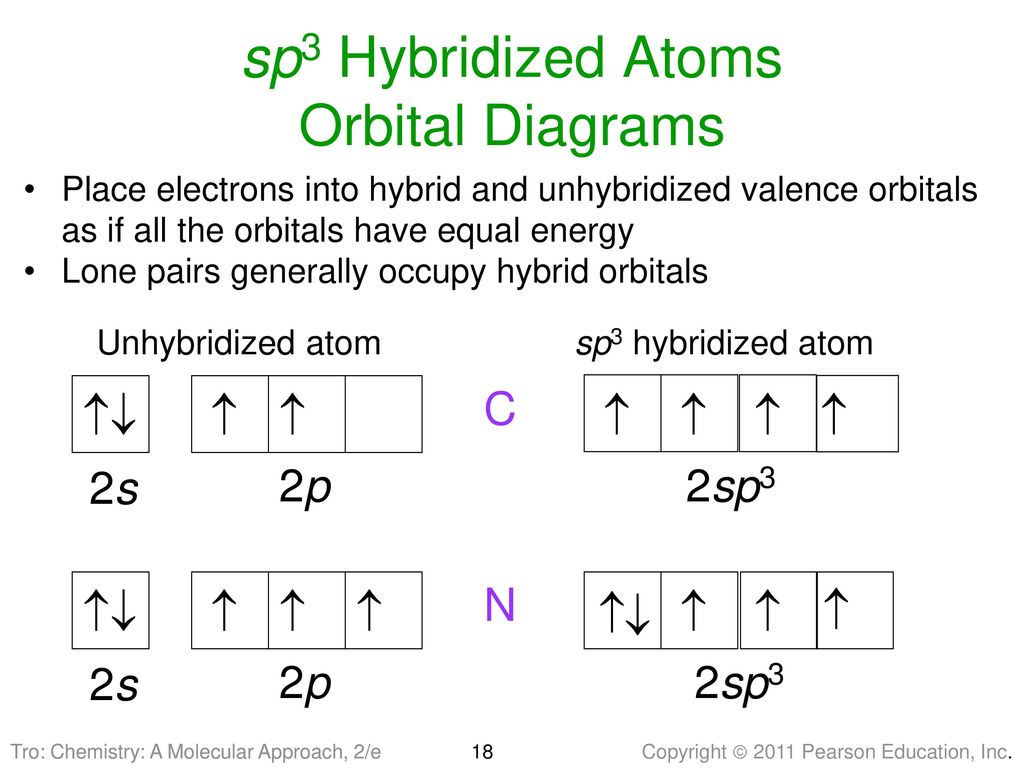
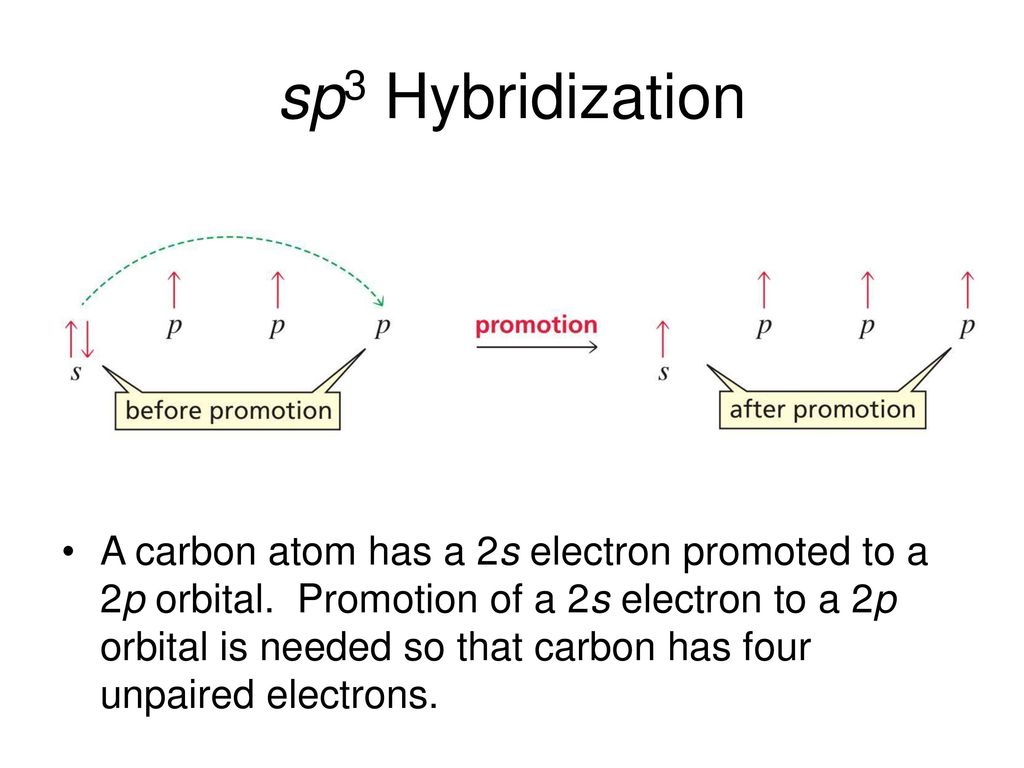
Orbital diagram of carbon before sp3 hybridization. Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized sp2 hybridization in ethene. In sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one p-orbital remaining. The two carbon atoms form a sigma bond in the molecule by overlapping two sp 2 orbitals. Solution for Draw orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. In the Lewis structure of CH3Cl, Carbon is at the central position and all the other atoms around it. The bond angles of Carbon with Hydrogen and Chlorine atoms are 109.5 degrees. This molecule has a tetrahedral shape, and the central carbon atom has sp3 hybridization. These "hybrid" orbitals are responsible for the two sigma bonds in BeCl 2 and are referred to as the "sp" hybrids. The orbital diagrams below show the"ground state" before bonding, the"excited state" ORBITAL PICTURE OF BONDING: ORBITAL COMBINATIONS The process that leads to their formation is called sp3 hybridization. 2s 2p X
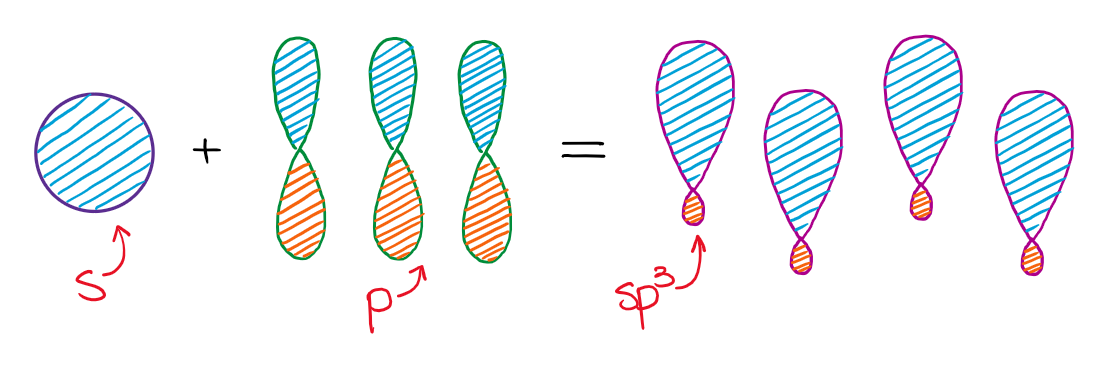
Shape of water molecule Lewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. Hydrogen bonds This unequal electron distribution results in strong non-bonding interactions between water molecules - hydrogen bonds. Answer (1 of 4): Carbon can form divalent compounds, and they are called carbenes, but they are rather reactive. The reason seems to be that if you have two bonds formed involving only p wave functions, the bonding, by forming electron pairs, creates a lot of electron density in a rather small vo... In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. 8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ...
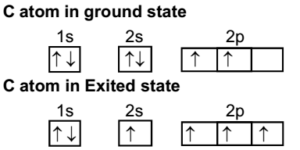
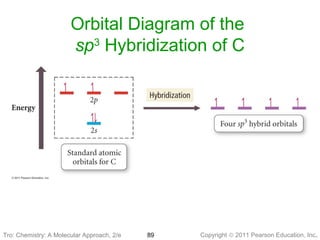
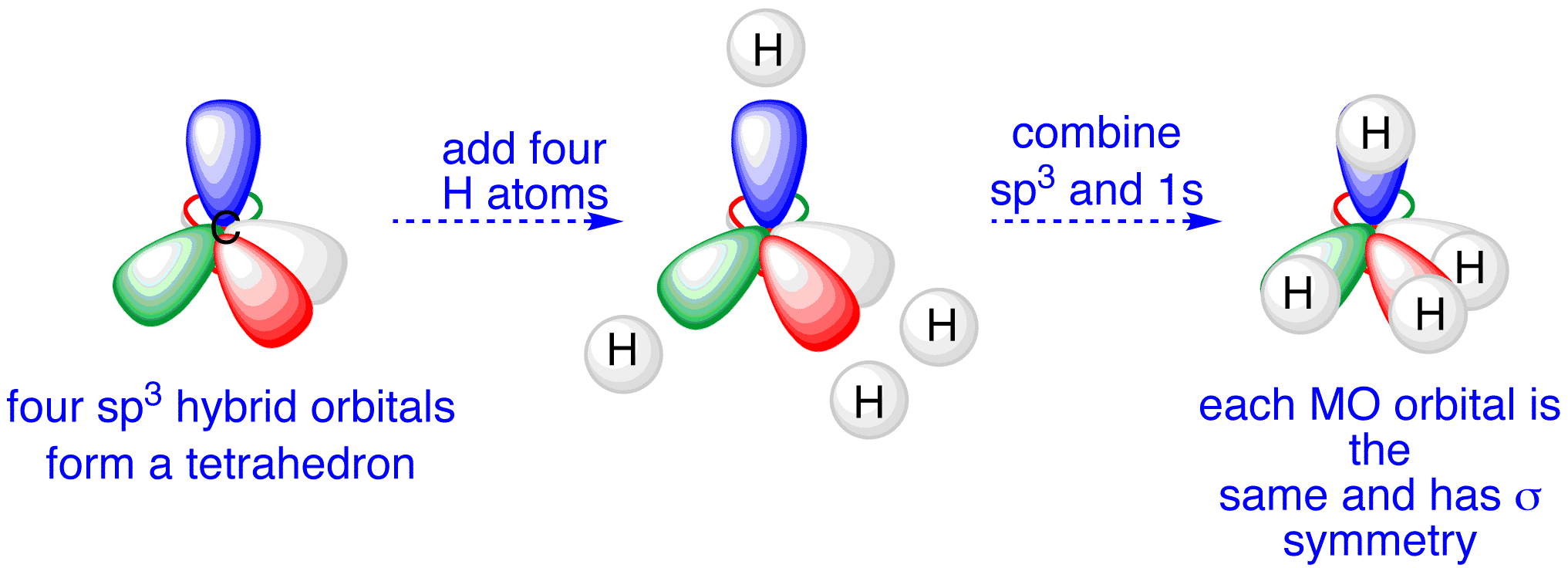
Write the orbital diagram of carbon before sp3 hybridization; Alabama vs texas a&m 2016 score; Y cb/pb cr/pr; Which feature of a bond contract allows the issuer Carbon (atomic number Z=6) in an unbonded state (ground state) has an electronic configuration of 1s2 2s2 2px1 2py1. The electrons in the 1s atomic orbital are ... This angle maximizes the distance between the orbital limbs, which is natural given that the electrons in each of the limbs repel one another. The shape of molecules like methane, CH4, with bond angles of 109.5°, is consistent with sp3 hybridization of carbon atoms. Here is an energy level diagram ... Explain the process of hybridization as it applies to the formation of sp3 hybridized atoms. ... The bonds in a methane (CH4) molecule are formed by four separate but equivalent orbitals; a single 2s and three 2p orbitals of the carbon hybridize into four sp3 orbitals.
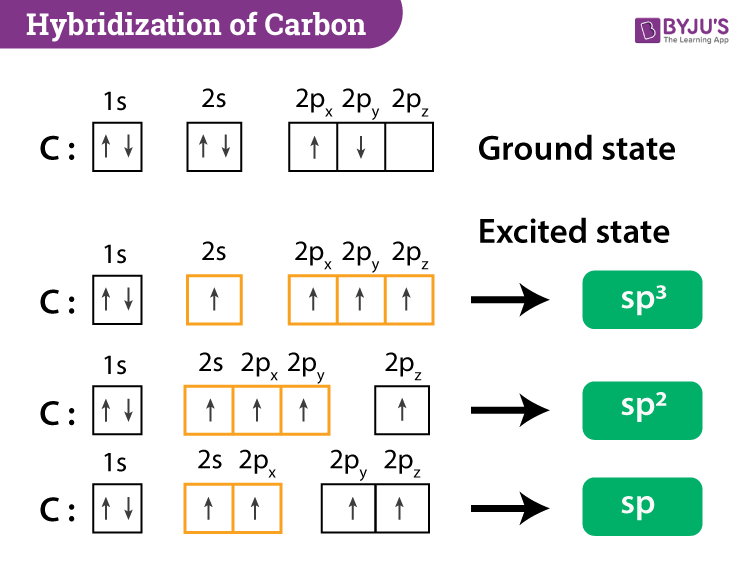
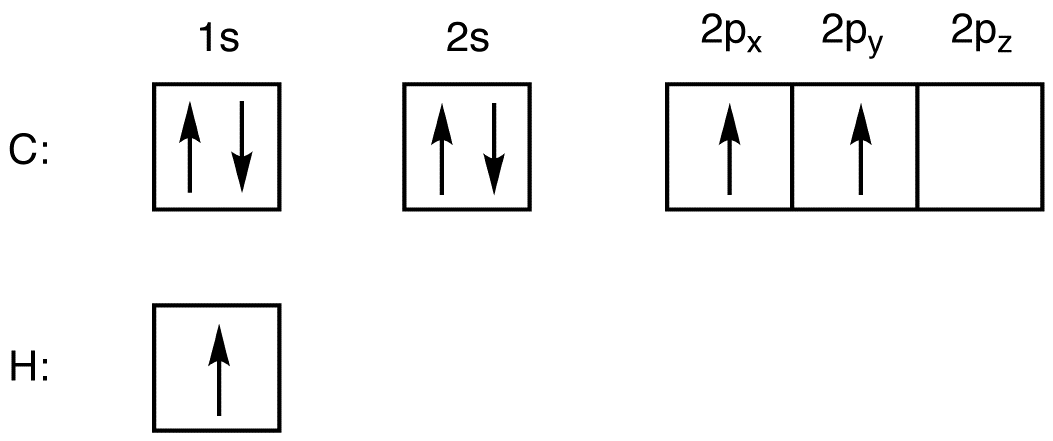
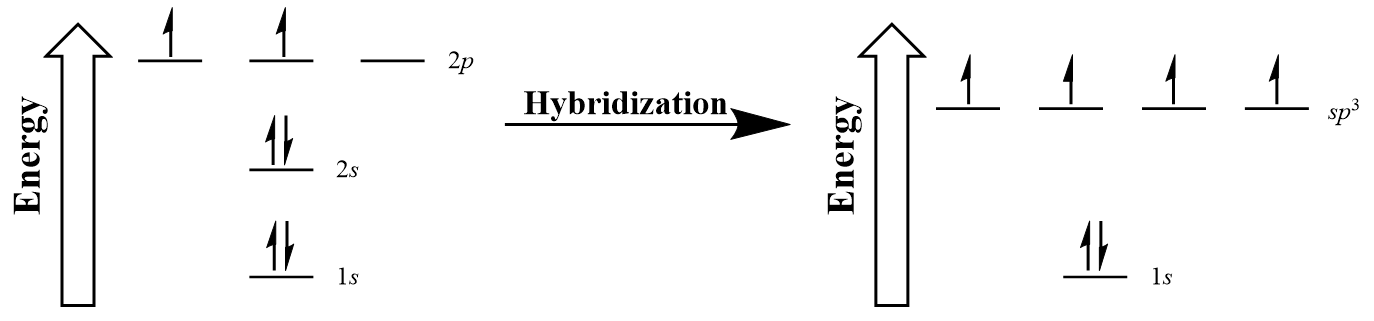
Let us draw orbital diagram to represent the electron configuration of carbon before and after s p 3 sp^3 s p 3 hybridization. The valence electron configuration: 2 s 2, 2 p 2 2s^2, 2p^2 2 s 2, 2 p 2.
The angle between the sp3 hybrid orbitals is 109.28 0; Each sp 3 hybrid orbital has 25% s character and 75% p character. Example of sp 3 hybridization: ethane (C 2 H 6), methane. sp 3 d Hybridization. sp 3 d hybridization involves the mixing of 1s orbital, 3p orbitals and 1d orbital to form 5 sp 3 d hybridized orbitals of equal energy. They ...
Orbital hybridisation - Wikipedia However to account for the trigonal planar shape of this BCl 3 molecule, sp 2 hybridization before bond formation was put forwarded. * In the excited state, Boron undergoes sp 2 hybridization by using a 2s and two 2p orbitals to give three half filled sp 2 hybrid orbitals which are oriented in …
orbital diagrams below show the"ground state" before bonding, the"excited state" Dec 26, 2021 · An 's' orbital will overlap with a 'p' orbital to create sp hybridization. If a central atom( here C) has two valence electron density regions surrounding it, then it
10/11/2018 · Just like in methane molecule, each carbon atom undergoes sp 3 hybridization in the excited state to give four sp 3 hybrid orbitals in tetrahedral geometry. * The two carbon atoms form a σ sp 3-sp 3 bond with each other …
The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite. 3. sp 3 Hybridization. When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent ...
orbital must become unpaired before they can bond. ... The bonds between the sp3 orbitals of hybridized carbon and the s orbitals.
Hybridization is used to explain molecular structures and describes the various orbital types which are involved in the bonding between atoms. Solution: Consider the electron configuration of a carbon schematron.org the orbital diagram of carbon before sp3 hybridization. Problem.
Write the orbital diagram of carbon before sp3 hybridization. Question: Consider the electron configuration. Write the orbital diagram of carbon before sp3 hybridization. This problem has been solved! See the answer See the answer See the answer done loading.
August 22, 2008 - Essentially, hybridisation is the ... form new orbitals – which can be used to describe bonding in molecules. Most importantly we have sp3, sp2 and sp hybridisation. ... The best way I can describe sp3 hybridisation is in Methane (also the most basic choice!). This is simplified for expression. Remember that Carbon has 6 ...
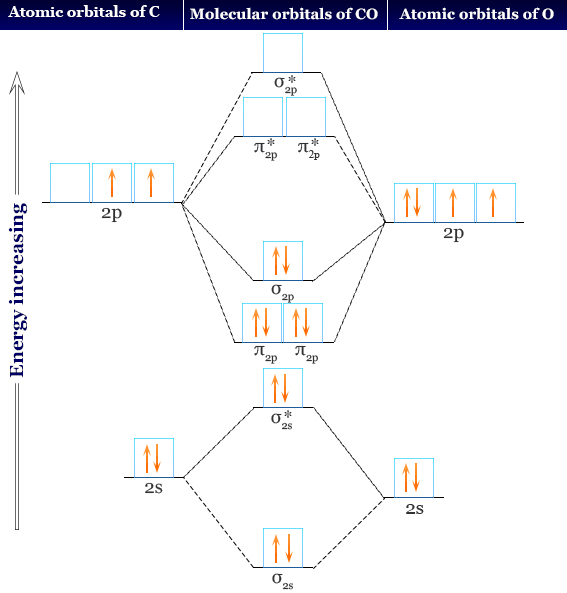
Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN¯. О 1.5 2p ´2p 2p 2 1 0.5 2.5 3 2s 2s 2.s Answer Bank 11 1 The atomic orbitals on the left side of the molecular The atomic orbitals on the right side of the molecular orbital diagram are those of orbital diagram are those of 9.
Carbon has an electron configuration of 1s^2 2s^2 2p^2. During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbitals. search. rotate. kaypeeoh72z and 14 more users found this answer helpful. heart outlined.
Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. orbital makes four, sp3 orbitals in a tetrahedral array. Molecular Orbital of Methane, CH4. 1. The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no .
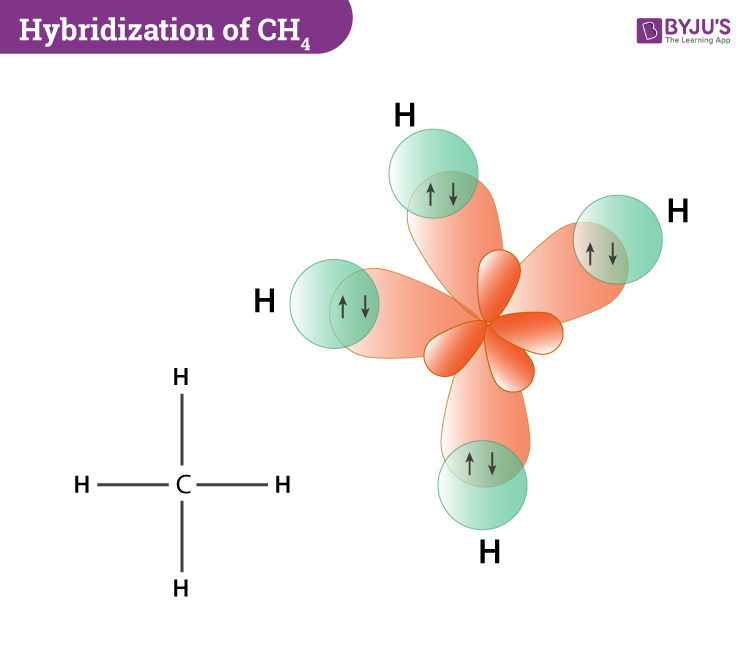
Each hybrid orbital contains one unpaired electron. v. Each of these sp 3 hybrid orbitals with one electron overlap axially with the 1s orbital of hydrogen atom to form one C-H sigma bond. Thus, in CH 4 molecule, there are four C-H bonds formed by the sp 3-s overlap. Diagram: Question G.
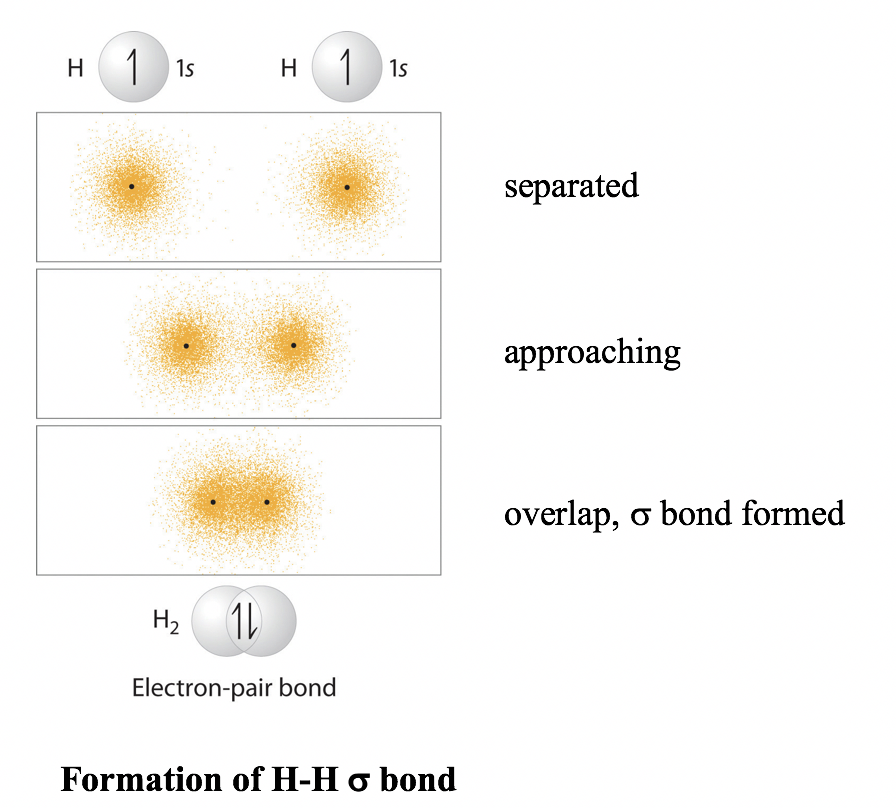
H2+ molecular orbital diagram Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π ...
After sp3 hybridization, the carbon atom has: a. a total of four unpaired electrons ... c. 1 σ bond forms between a hybrid sp orbital on C and an s orbital on H; 1 σ bond forms between a hybrid sp orbital on C and a hybrid sp orbital on N ... Molecular Orbital Diagram-Shows the atomic orbitals of the atoms, the molecular orbitals of the ...
2 weeks ago - When we say, methane is sp3 hybridized, we mean the carbon atom in methane is sp3 hybridized, not methane as a whole or four hydrogen atoms. The electronic configuration of carbon in the ground state is [He] 2s2 2p2. This can be better understood from the below diagram. Each orbital occupies ...
By the obsolete concept of hybridization, all carbon atoms in graphite are sp 2 hybridized. A total of three (1 - 2s and 2 p-orbitals) lead to this hybridization. Due to the formation of sigma bonds with axial overlap of one of these sp 2 orbitals with another sp 2 orbital of another centre, the atoms exist as a planar array of hexagonal carbon ...
Draw orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization.
Hence four hybrid orbitals are formed for CH 4, and referring to the table given below, we can say that it has sp3 hybridization. One 2s orbital and three 2p orbitals are hybridized for the Carbon atom. CH4 Molecular Geometry. Molecular geometry helps us understand the arrangement of atoms in 3D for any given molecule.
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. FREE Expert Solution. Before hybridization, we have: 100% (117 ratings) Problem Details.
Orbital Diagram Of Carbon Before Sp3 Hybridization This makes the central carbon atom sp3 hybridized where the three hydrogen atoms are s-sp3 hybridized and the single chlorine atom is sp3-p hybridized. Hybridization CH3OH Shape. loop Diagram Axes for you bonding in methane - sp3 hybridisation
Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Q. Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics.
14/03/2016 · Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital (s) from the other, incoming atom (s). You can read more about sp3 hybridization here. The qualitative energies turn out to be the following:
Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Q. Select the correct hybridization for the central atom based on the electron geometry CCl4.
So instead of this being a 2s, ... like for carbon is that this looks like a 2sp3 orbital. This looks like a 2sp3 orbital, that looks like a 2sp3 orbital, that looks like a 2sp3 orbital. They all look like they're kind of in the same orbital. This special type of-- it sounds very fancy. This sp3 hybridized orbital, what ...
Re: Ethene sp^2 Hybridization. A good bookkeeping strategy would be to acknowledge the number of regions of electron density around an atom. In your case for ethene, , a carbon atom has three regions of electron density (a single bond, a single bond, and a double bond). Since there are three regions of electron density, if we were to apply ...
Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like.
The remaining sp hybrid orbital of each carbon atom overlaps with the ls orbital of hydrogen atom along the internuclear axis to form two sigma \((\sigma)\) bonds. Each carbon atom has two unhybridised 2p orbitals which are perpendicular to each other as well as to the plane of the C-C bond.
Type of Hybrid Orbital in a Single Species: For much nicer three-dimensional renderings of all the hybrid orbitals visit Mark Winter's Oritron site . The symmetry operations are shown interactively in 3D Remember, the f orbital has to included before the d orbital because bismuth is after the lanthanide series. helium, Z = 2 c. krypton, Z = 36 neon, Z = 1 0 d. xenon, Z = 54 IF 4 â and the ...
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization Pre-requisite Reading- Valency the Elements, modern-day Electronic configuration, atomic orbitals, ide of Hybridization Of the three states of hybridization - sp3, sp2, and also sp, one sp3 (pronunciation: ess-pee-three ) hybridization ...
March 2, 2021 - 2. Draw the energy diagram for the orbitals of sp3 hybridzied carbon and nitrogen. Then fill in the correct number of electron. 3. Indicate the hybridization of oxygen in each molecule ... 2. Just like the energy diagram in fig.3. For carbon, each sp3 orbital has 1 electron.
Hybridization occurs when bonding to allow the orbitals that need to be in place to occur and explain the observed structures. In class, it was mentioned that seeing the regions of electron density will equal the number of hybrid orbitals, so if you know that there is two regions of density, you will know the hybrid needs two orbitals and is therefore sp because there is one s orbital and one ...
Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron ...
An explanation of the bonding in methane and ethane, including a simple view of hybridisation
Q. Draw orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization. Solved • Sep 29, 2021 Hybridization Q. Please explain the hybridization of carbon in C2H4(sp2) and C2H2(sp). Solved • Sep 8, 2021 ...





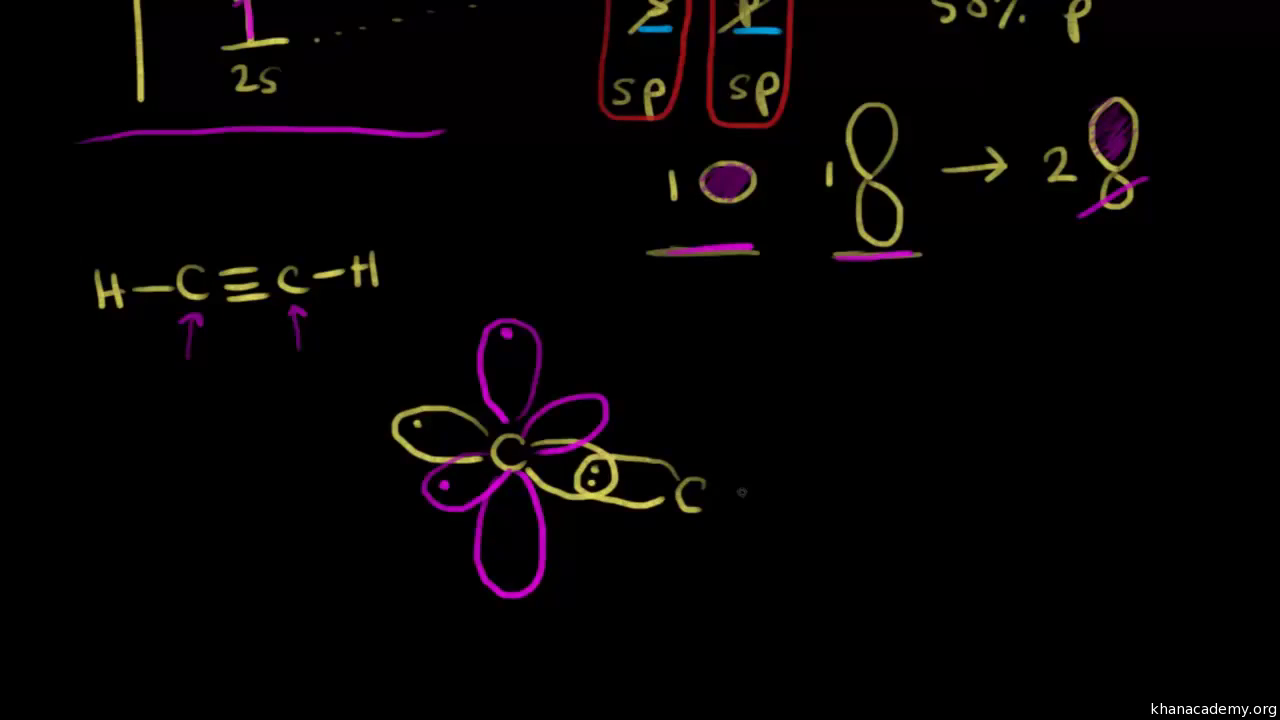






![Hybridization [sp3, sp2, sp orbitals] Flashcards | Quizlet](https://o.quizlet.com/IJGVT7le9W6sz4PHrnk3IQ.png)


0 Response to "36 orbital diagram of carbon before sp3 hybridization"
Post a Comment