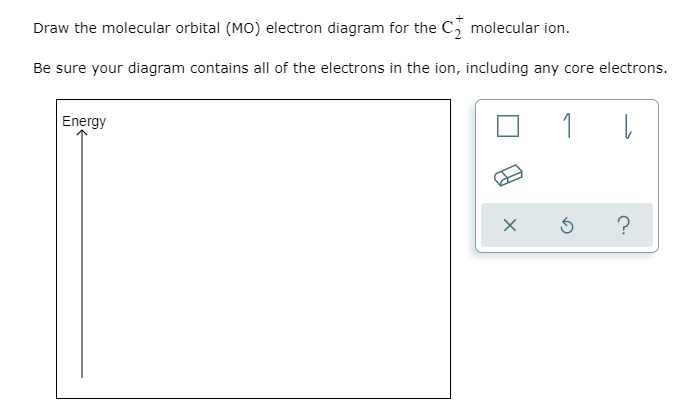
38 C2+ Molecular Orbital Diagram
Solved 2. a) Sketch and label the bonding and antibonding ... 2. a) Sketch and label the bonding and antibonding combinations of s and p orbitals overlapping. [1 mark] b) Draw the molecular orbital diagram of C2-3 and complete this with the appropriate number of electrons and any appropriate labelling. [6 marks] c) (1) Calculate the bond order of CO+1. Is the molecule stable? [2 marks] (ii) The electronic ... 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
PDF Using Symmetry to Generate Molecular Orbital Diagrams atomic orbitals a 1g (σ g) 2s e 1u (π u) p y p x p z a 1u (σ u) atomic orbitals of terminal atoms H 1 + H 2 LGO (1) LGO (2) a 1u (σ u) …..thus, the symmetry of LGO(2) matches that of the X atom's p z - orbital …..and LGO(2) will combine with the X p z -orbital to form a new molecular orbital (MO) …..this interaction is symmetry ...
C2+ molecular orbital diagram
Solved The following is the molecular orbital diagram ... The following is the molecular orbital diagram showing the energy ordering for the molecular orbitals. Use this diagram to answer the question. Atomic orbitals Molecular orbitals Atomic orbitals 22 2p 2p Energy 2s 25 B2, C2, N2 Part A Which of the following has the shortest bond length? Answered: molecular orbital diagram for C2 | bartleby Solution for molecular orbital diagram for C2. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. Start exploring! Subjectschevron_right ... PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
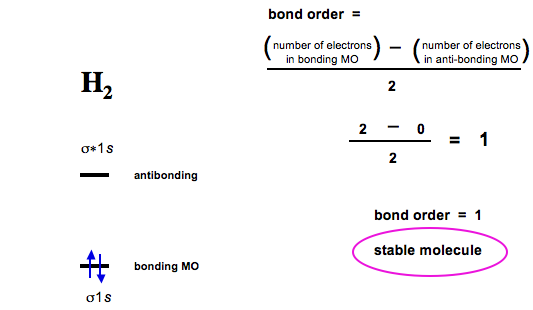
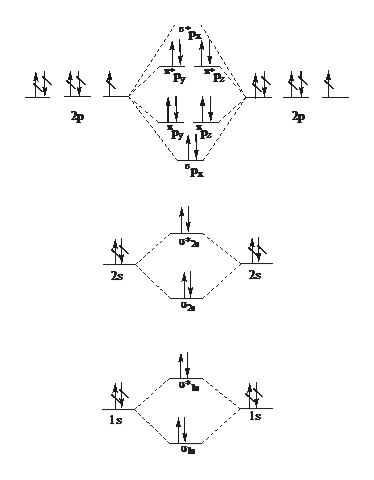
C2+ molecular orbital diagram. Bond order of c2+ ,c2 2- - Brainly.in Bond order of C2- = 1/2 (7 - 2) = 5/2 = 2.5 Bond order of C2 = 1/2 (6 - 2) = 2 Highest bond order means highest bond energy and shortest bond length. So, the highest bond order with highest bond energy and the shortest bond length is found in C2-. So, the order starting with the highest bond order is = C2- > C2 > C2+. Molecular Orbital (MO) Diagram for C2(2-) - YouTube When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i... Li2- Molecular Orbital Diagram Answer to Draw a molecular orbital energy diagram for Li2. What is the bond order? Is the molecule likely to be stable? Explain. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. 2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +.Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
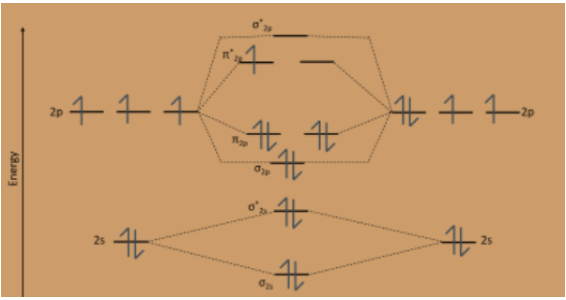
Molecular Orbital C2- Diagram, Bond Order, Magnetism ... Molecular Orbital C2- Diagram, Bond Order, Magnetism. Postby Brooke Tobias 1B » Fri Nov 27, 2015 7:31 pm. A video explaining the molecular orbital diagram and notation of C2- along with the bond order and magnetism. You do not have the required permissions to view the files attached to this post. Top. N2+ Mo Diagram - schematron.org Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. 6.9: Summary of Molecular Orbital Theory - Chemistry ... Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
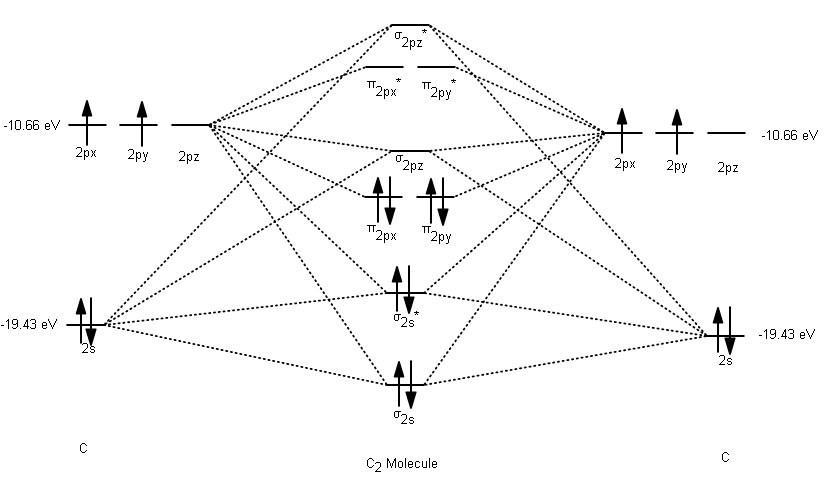
C2 molecule, doubly or quadruply bonded? - Mapping Ignorance The formal bond order calculated with these orbitals and occupation numbers is 2 (resulting from 6 electrons in bonding orbitals and 2 in an antibonding orbital). Figure 2. Schematic picture of the molecular orbital diagram obtained from MO theory. In VB, the bond picture arises from considering that the C atom bears a sp hybridization. write molecular orbital configuration of c2 predict ... The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. C22- Molecular Orbital Diagram Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Mar 26, · This video shows the MO diagrams of the C2, N2, O2 and F2 molecules.A molecular orbital (MO) energy level diagram - Parkway C-2Use the molecular orbital ... Molecular Orbital Diagram of B2, C2, and N2 Molecules ... From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
C22- Molecular Orbital Diagram - schematron.org on C22- Molecular Orbital Diagram. The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. It is sigma2s (2)sigma2s* (2)sigma2p (2)pi2p (4)pi2p* (4) Bond order 1. It is stable. In fact, it's the perioxide ion. Check me out.
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
DOC Molecular Orbitals of Diatomic Molecules - La Salle University This diagram should be to scale. If necessary, you can omit the two lowest molecular orbitals from the diagram. Write the molecular orbital configuration for your molecule. Calculate the bond order for your molecule. Jen B2 Jeff OH- Mike F C2 Laura Li2 Jill Be2 Cody N2 Diana CO Kevin BF Wai BH Mike S NH Ly HF Jake SH Melissa F2
What is the molecular orbital diagram for C2 class 11 ... Molecular orbital theory shows that it has two sets of paired electrons in a degenerate bonding set of orbitals. This gives a bond order of two, which means that there should exist a double bond between the two carbons in a C 2 . As you know, a neutral carbon atom has a total of six electrons. This, of course, implies that a C 2 molecule has a ...
what is the bond order of c2+? - Lisbdnet.com 37 Molecular orbital diagram and bond order for C2 What Is The Bond Order Of C2+?? The bond order of C2 molecule is 2. What is the bond order C2+? Bond order = 1/2 (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) Therefore, Bond order of C2+ = 1/2 (5 - 2) = 3/2 = 1.5. Which is more stable C2 or C2+?
PDF ElectronicstructureconsiderationsforC2 andO2 Millard H ... FIG. 1. The 1σg (left panel) and 1σu (right panel) molecular orbitals of C2 The next two molecular orbitals (2σg and 2σu) corresponds to the symmetric and, re-spectively, antisymmetric linear combinations of the 2s orbitals on each C atom. Note that the 2σu orbital also contains a substantial contribution from the 2pz (l = 0) orbitals (the 2p
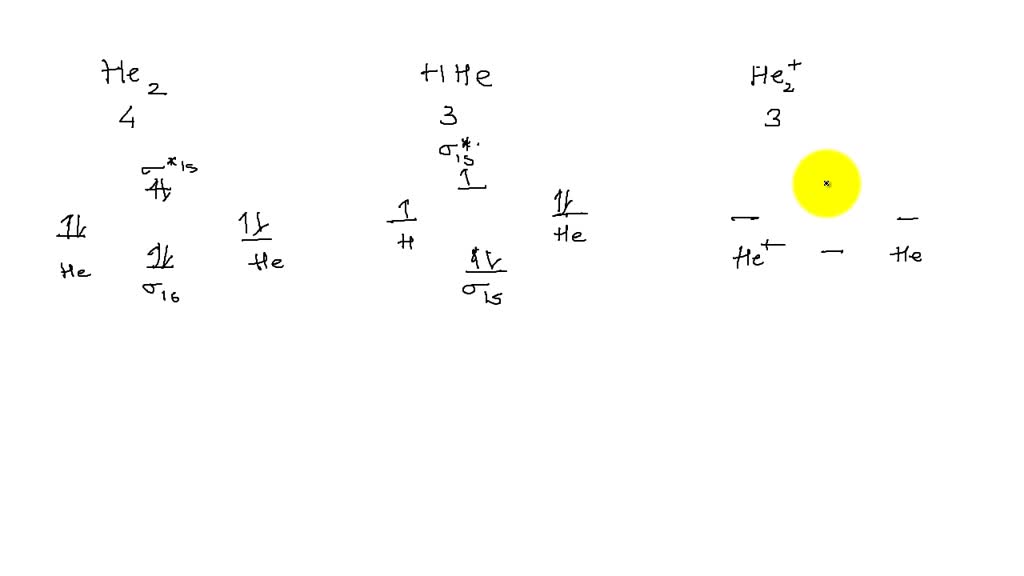
draw molecular orbital diagram for c2 n2 o2 and he2 and calculate bond order and predict the manganic properties for each molecules
How to predict the existence of Li2 C2 with their ... - Quora Answer: This is a very ambitious calculation. F. A. Matsen did such a calculation on the molecule Li-H about fifty years ago, but the calculations have become more streamlined since then. The results from such calculations will not actually predict the "existence" of Li2C2, but will provide a re...
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Answered: molecular orbital diagram for C2 | bartleby Solution for molecular orbital diagram for C2. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. Start exploring! Subjectschevron_right ...
Solved The following is the molecular orbital diagram ... The following is the molecular orbital diagram showing the energy ordering for the molecular orbitals. Use this diagram to answer the question. Atomic orbitals Molecular orbitals Atomic orbitals 22 2p 2p Energy 2s 25 B2, C2, N2 Part A Which of the following has the shortest bond length?






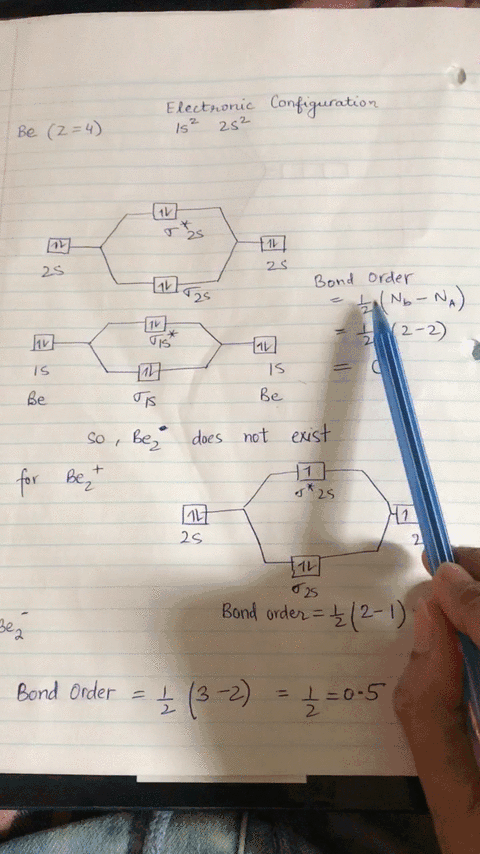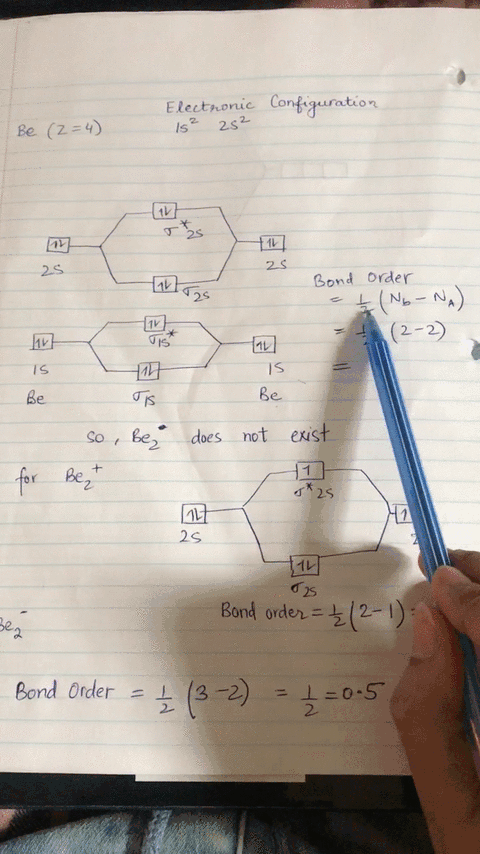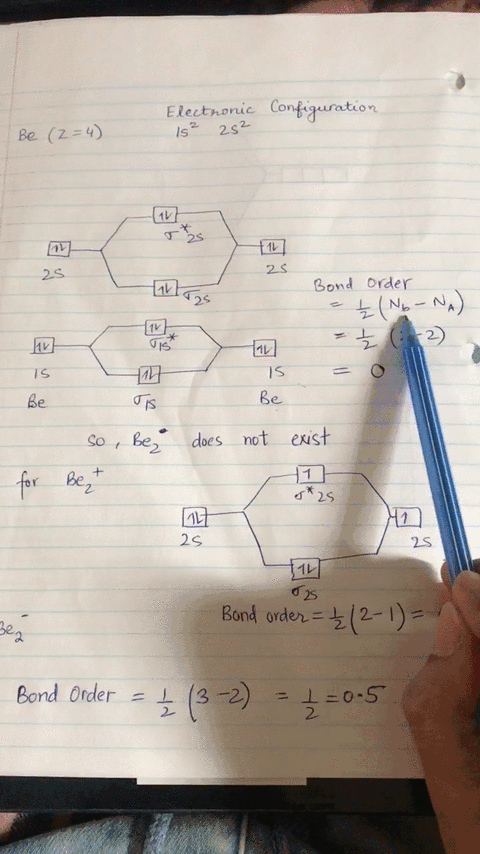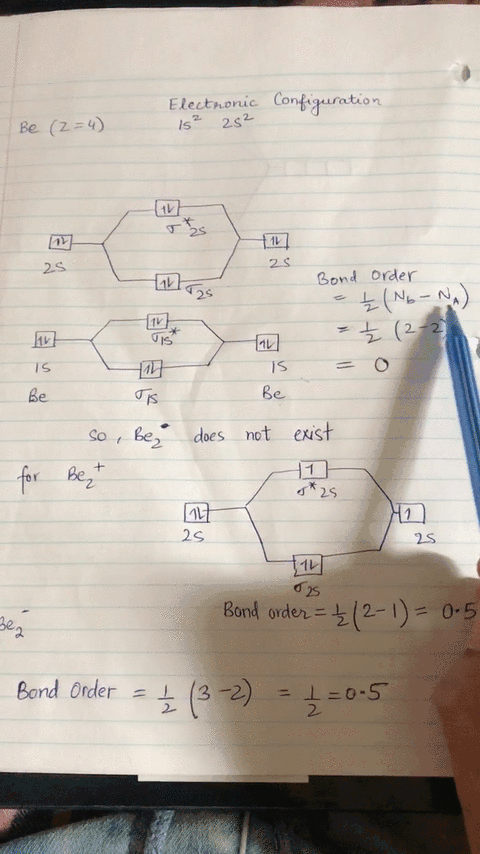




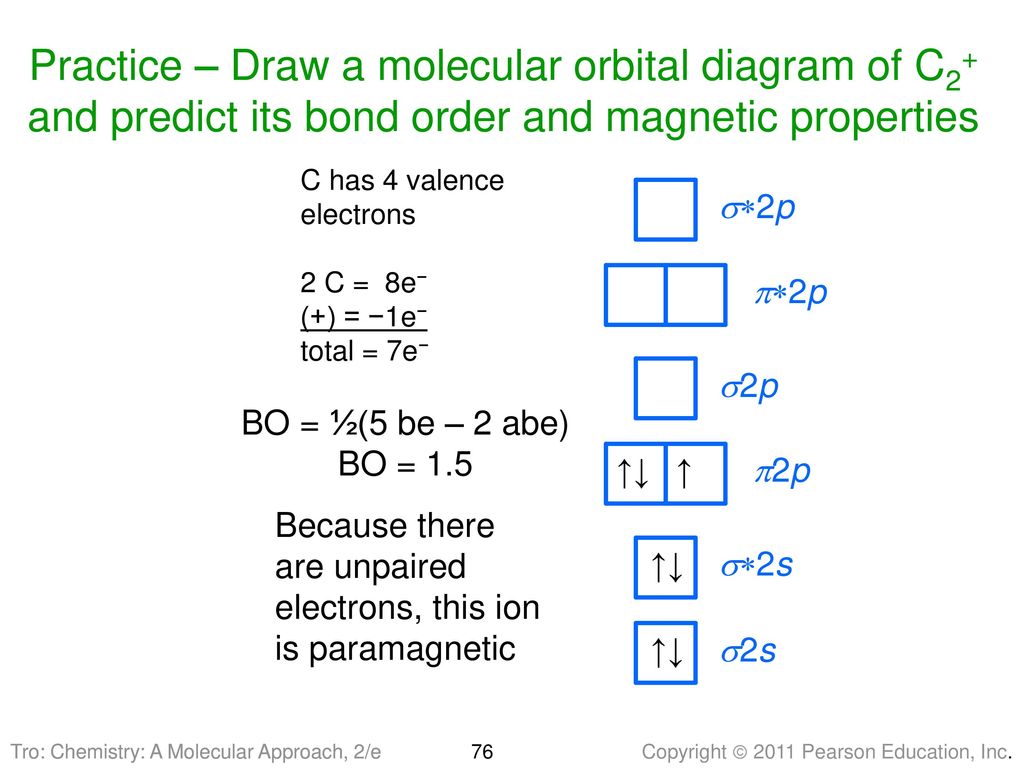



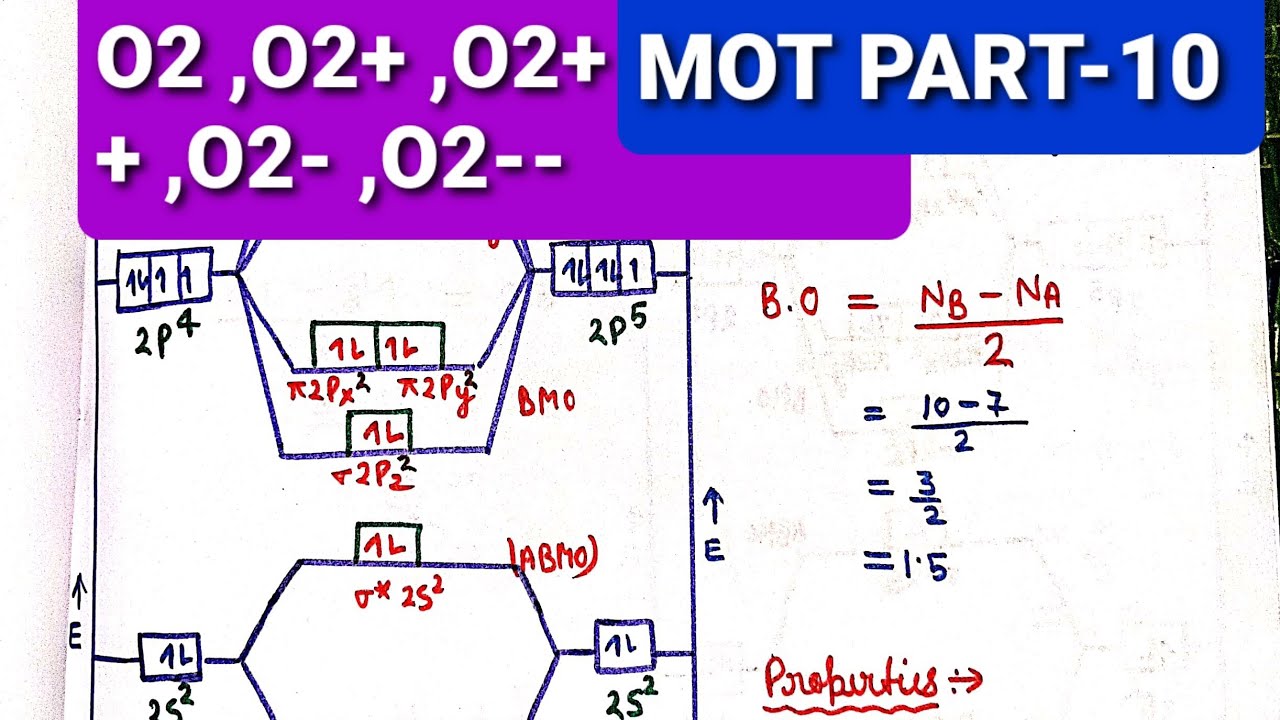
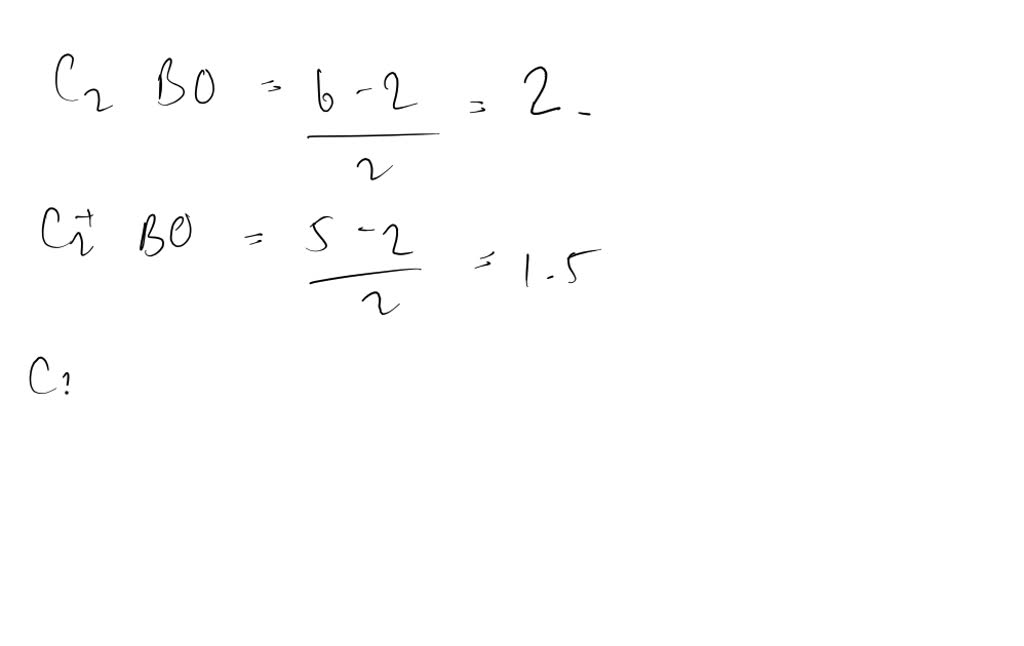
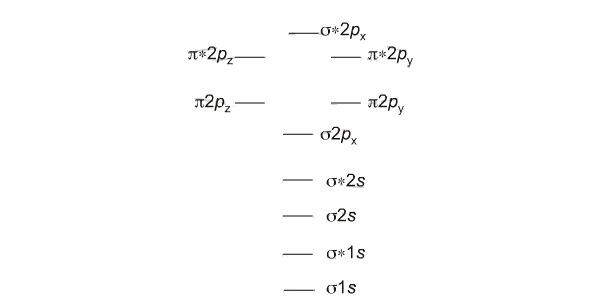




0 Response to "38 C2+ Molecular Orbital Diagram"
Post a Comment