38 cf molecular orbital diagram
8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... CF4 Tetrafluoride Lewis Structure, Molecular Structure ... CF 4 comprises a Carbon atom surrounded by four Fluorine atoms. In its most stable state, the Carbon atom forms covalent atoms with the Fluorine atoms. There are no lone pairs. The hybridization of the CF 4 is given by sp 3. CF 4 has a Tetrahedral molecular structure and shape with bond angles of 109.5 °.
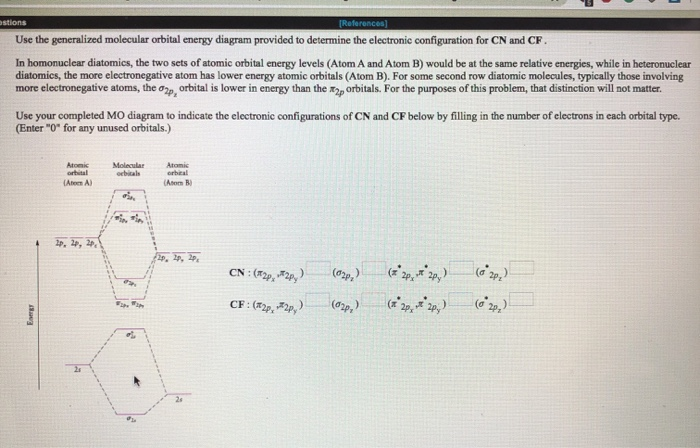
MO Theory: Heteronuclear Diatomic Molecules - Clutch Prep Practice: Apply Molecular Orbital Theory to determine the MO orbital diagram for the CF + ion. Practice: Using a MO diagram, write the electron configuration for the BN molecule? Progress. 0 of 4 completed. Videos in MO Theory: Heteronuclear Diatomic Molecules.

Cf molecular orbital diagram
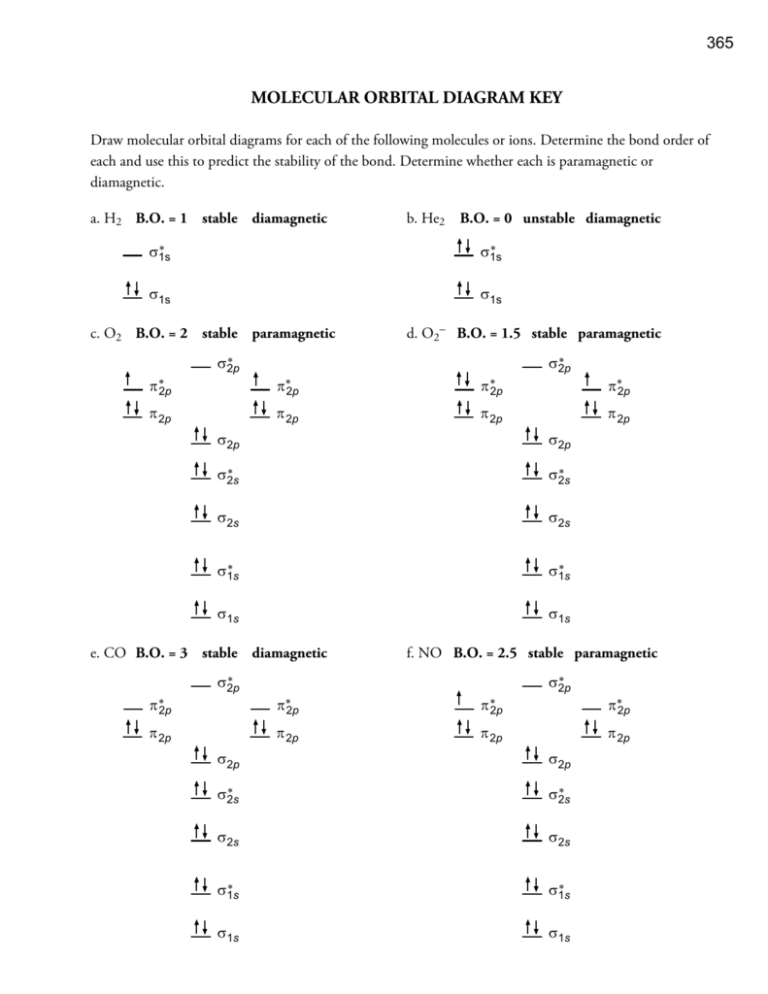
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ... What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. PDF Finding the HOMO and LUMO - Michigan State University Predicting Reactions Using Frontier Orbitals • Here are two species you have never seen before. Using Frontier Molecular Orbital Theory (FMO Theory), predict the first step of the reaction between these two species. Draw the curved-arrow mechanism that shows how they react, and predict the immediate product of that reaction.
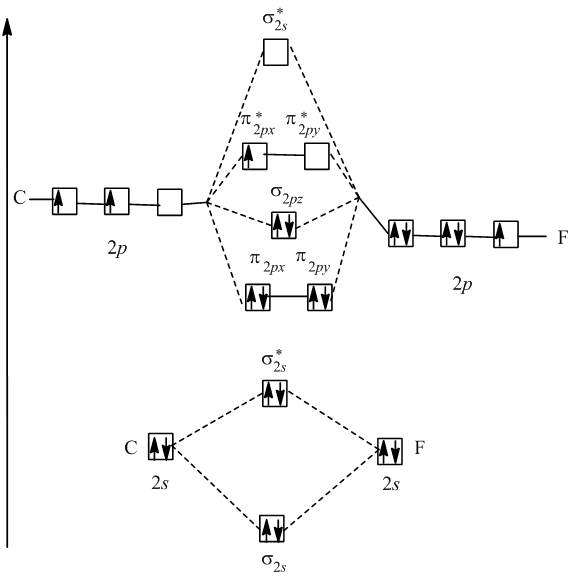
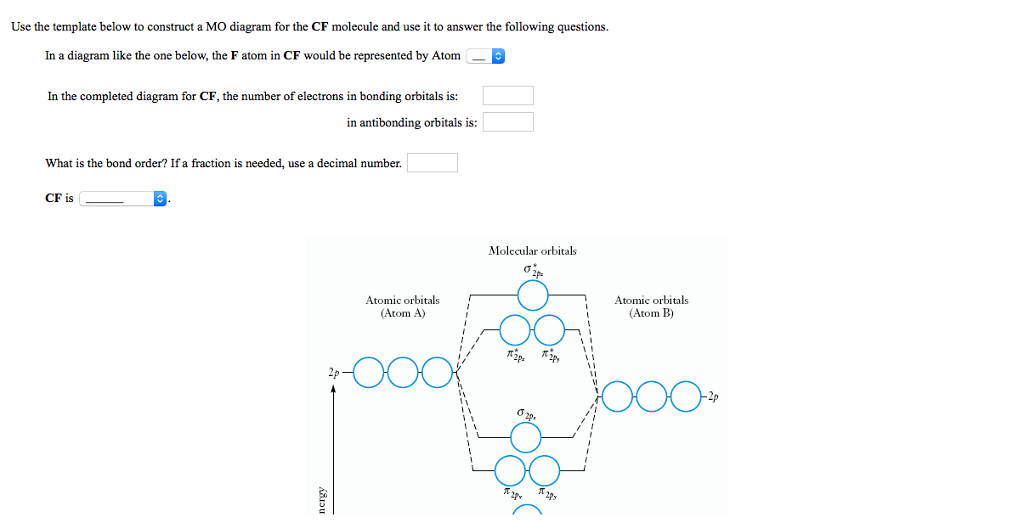
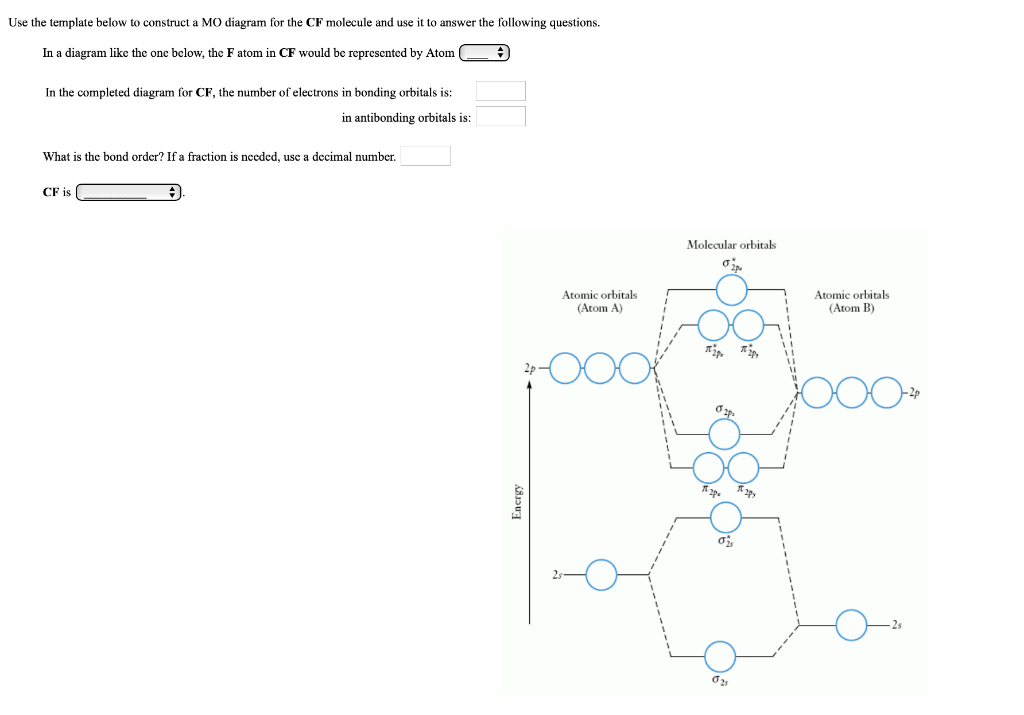
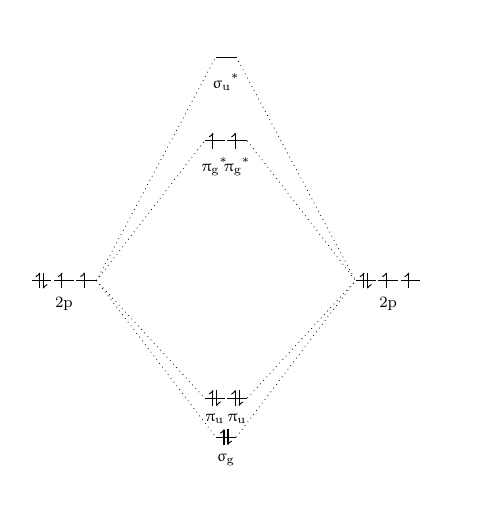
Cf molecular orbital diagram. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Solved Question 7 3 points Save A Draw the molecular ... Question 7 3 points Save A Draw the molecular orbital diagram (MO diagram) for the heteronuclear diatomic species CF to answer the following questions. Only show the valence Atomic orbitals and valence Molecular orbitals. The order of MO energy levels for CF will be the same as for the O2 homonuclear diatomic molecule. Frontier Molecular Orbital Theory Explained - HRF Frontier molecular orbital theory is an application of the MO theory that describes the interactions of HOMO and LUMO interactions. First published in the Journal of Chemical Physics by Kenichi Fukui in 1952, it is a theory of reactivity that would eventually help Fukui share a Nobel Prize in Chemistry for reaction mechanisms.. He would become the first Asian scientist to win a chemistry-based ... On the Origin of Covalent Bonding in Heavy Actinides A modest increase in measured actinide:dipicolinate stability constants is coincident with a significant increase in An 5f energy degeneracy with the dipicolinate molecular orbitals for Bk and Cf relative to Am and Cm.
Molecular Orbitals - an overview | ScienceDirect Topics Such formulae enable us to calculate the contributions of the particular donor-acceptor resonance structures (e.g., DA, D + A −, etc., cf. p. 520) in the Slater determinants built of the molecular orbitals (14.37) of the total system. If one of these structures prevailed at a given stage of the reaction, this would represent important information about what has happened in the course of the ... PDF authors.library.caltech.edu authors.library.caltech.edu PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the PDF MO Scheme of BF - University of Massachusetts Boston orbitals, is Ö = c 1 ö(B) - c 2 [ö(F a) + ö(F b) + ö(F c)] • The LCAO for this is! For the base NH 3, the HOMO is the weakly bonding ó a 1 MO (cf. MO scheme), which can provide electrons to the boron ð* 2a 2 " MO in the following manner:
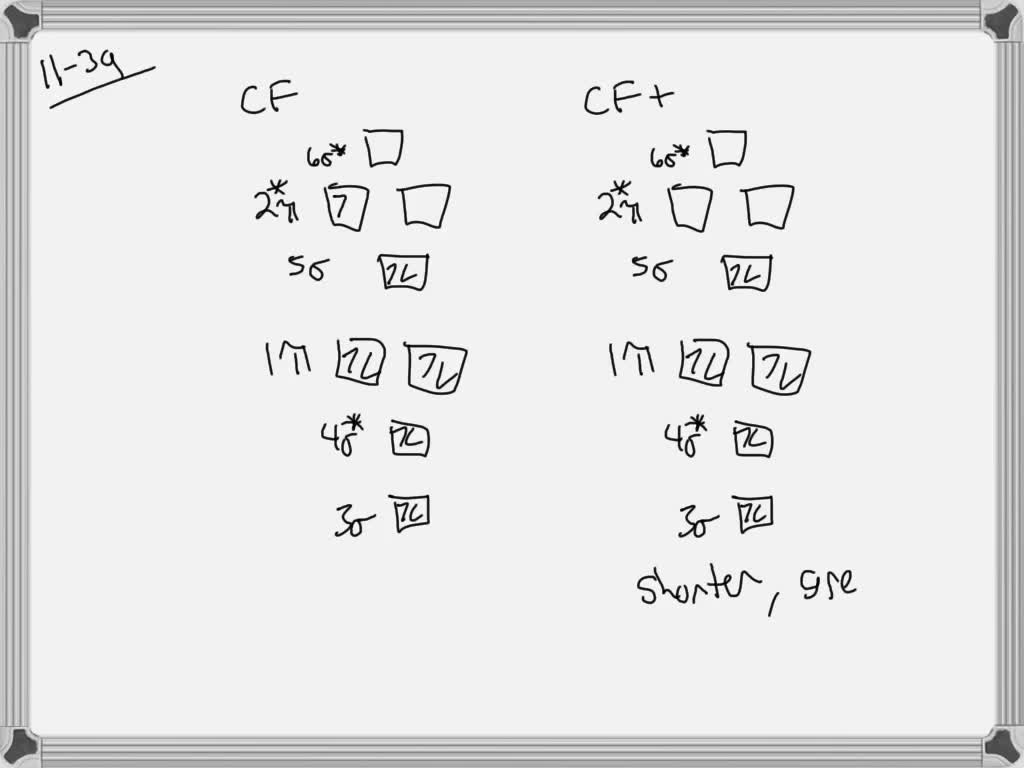
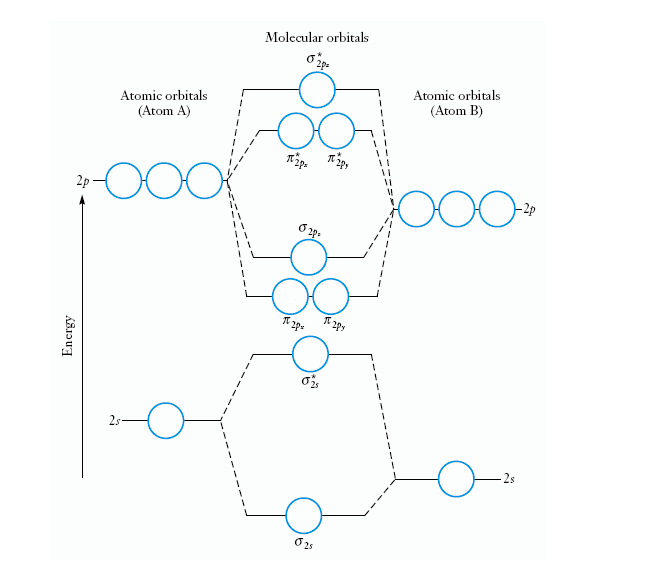
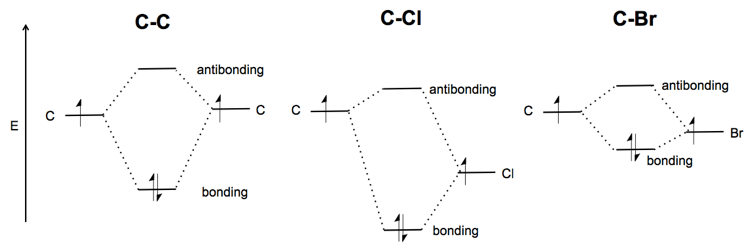
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... PDF 2 Atomic and Molecular Orbitals - Washington University in ... Atomic and Molecular Orbitals 2.1 Atomic Orbitals According to quantum mechanics, an electron bound to an atom cannot possess any arbitrary energy or occupy any position in space. These characteristics can be deter-mined by solving the time-independent Schrödinger equation: Hϕϕ E (2.1) where H is the Hamiltonian operator of the atom. SOLVED:Shown below is the molecular orbital diagram for Oz ... to find out which bond is going to be shorter, CF or CF plus me to find out the difference in their molecular orbital configurations. Turns out that the difference is with CF plus shown here on the right, we have one fewer electrons. When the one fewer electron came from an AN or was removed from an anti bonding molecular orbital. Any time we remove an electron from an anti bonding molecular ... MO for CF have pi orbital below sigma? - CHEMISTRY COMMUNITY The MO for CF has the pi orbitals below the sigma orbital. For the heteronuclear diatomic molecules where one atom has Z < 8, the pi orbitals are usually below the sigma orbital like NO and CO. Top. 2 posts • Page 1 of 1. Return to "*Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)".
Solved: Chapter 11 Problem 39E Solution | Selected ... - Chegg MO diagram of CF molecule is as follows: The MO diagram shows various molecular orbitals present in the CF molecule. The MO orbitals that have lower energy are bonding MO orbitals and the MO orbitals that have higher energy are called antibonding orbitals. Chapter 11, Problem 39E is solved. View this answer View a sample solution Step 2 of 5
Ch2 MO Theory - DocShare.tips Molecular Orbital diagram fro N2 Bond Order The number of bonds between a pair of atoms is called the bond order. Bond orders can be calculated from Lewis structures, which are the heart of the valence-bond model. ... In CF 4 the C-F bondlength is 1.32 Å (predicted 1.44 Å) In SiF 4 the Si-F bond length is 1.54 Å (predicted 1.81 Å)
Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
Molecular Orbital - an overview | ScienceDirect Topics Molecular Orbital. Molecular orbital calculations, which have been carried out for 1,2,3,4,5-pentathiepin (62), 1,2,3,4,5-benzopentathiepin (35), and thieno[3,4-f]-1,2,3,4,5-pentathiepin (37) by means of the semiempirical AM1 method 〈88JST(167)245〉 and for the pentathiepin by means of ab initio method 〈87JOC2411〉 show the same order of the stability for the different conformers.
Molecular Orbital Investigation of CF and SiF and Their ... Accurate Hartree-Fock self‐consistent‐field wavefunctions have been computed for CF, CF +, and CF − at several internuclear separations by the Roothaan expansion method. Similar wavefunctions were also computed for SiF, SiF +, and SiF − at the parent molecule internuclear separation. A Dunham analysis of the energy curves for the three CF species yielded values for the spectroscopic ...
Finding bond length for CF-, CF and CF+ - CHEMISTRY COMMUNITY Based on the MO diagram, CF has a bond order of 2.5. In CF+, an electron is taken off from pi 2p orbital (which is the highest occupied orbital), bond order becomes 3. In CF-, an electron is added to pi 2p orbital and bond order becomes 2. So CF- should have the longest bond. Top 2 posts • Page 1 of 1
How do I correspond the structure of "SO"_2 to a molecular ... "SO"_2 is a bent structure (molecular geometry). Sulfur wants 6 electrons, and so does oxygen. Hence, 6xx3 = 18 valence electrons to distribute throughout the structure. Putting 4 for each of two double bonds uses up 8. You can then put 4 electrons on each oxygen and the last 2 on the sulfur as a lone pair. Sulfur can be hypervalent (cf. "SF"_6), but oxygen cannot because sulfur can access its ...
SOLVED:Construct the molecular orbital diagram for CF ... Construct the molecular orbital diagram for CF. Would you expect the bond length of $\mathrm{CF}^{+}$ to be longer or shorter than that of CF? Answer. To both CF and CF' ions, CF' has shorter bond length than CF. This is because CF' bond order more than CF molecule. View Answer.
FIG. 3. Orbital diagrams for (a) the CF ( X 2 Π ) state ... The corresponding GVB orbital diagram is given in Fig. 3(a). In the separated atom limit (top left), the carbon (2s − , 2s + ) lobe orbitals are singlet coupled, the carbon (2p x , 2p z ...
Answered: What is the likely formula of potassium… | bartleby Draw a molecular orbital diagram and explain. Expert Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!*
PDF Chem 344 - Homework 9 due Friday, Apr. 11, 2014, 2 PM P16.18) Images of molecular orbitals for LiH calculated using the minimal basis set are shown here. In these images, the smaller atom is H. The H1s AO has a lower energy than the Li2s AO. The energies of the MOs are (left to right) -63.9, -7.92, and +2.14 eV. Make a molecular orbital diagram for this molecule, associate the MOs with the images,
PDF Finding the HOMO and LUMO - Michigan State University Predicting Reactions Using Frontier Orbitals • Here are two species you have never seen before. Using Frontier Molecular Orbital Theory (FMO Theory), predict the first step of the reaction between these two species. Draw the curved-arrow mechanism that shows how they react, and predict the immediate product of that reaction.
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
![Molecular Orbital Theory (MOT), Bonding in [Fe(CN)6]4– ion ...](https://ebrary.net/htm/img/33/1908/304.png)














0 Response to "38 cf molecular orbital diagram"
Post a Comment