39 Orbital Diagram Of Aluminum
What is the orbital diagram of aluminum? - Quora Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. Orbital Diagram For Calcium (Ca) | Calcium Electron ... Are you searching for Calcium Electron Configuration (Ca) with Orbital Diagram? Every person should learn about the chemical Orbital Diagram, electronic configuration, atomic number, atomic mass, molecules, etc. It will not only be necessary for chemistry subjects, but it is also essential for general knowledge. If you are a chemistry student ...
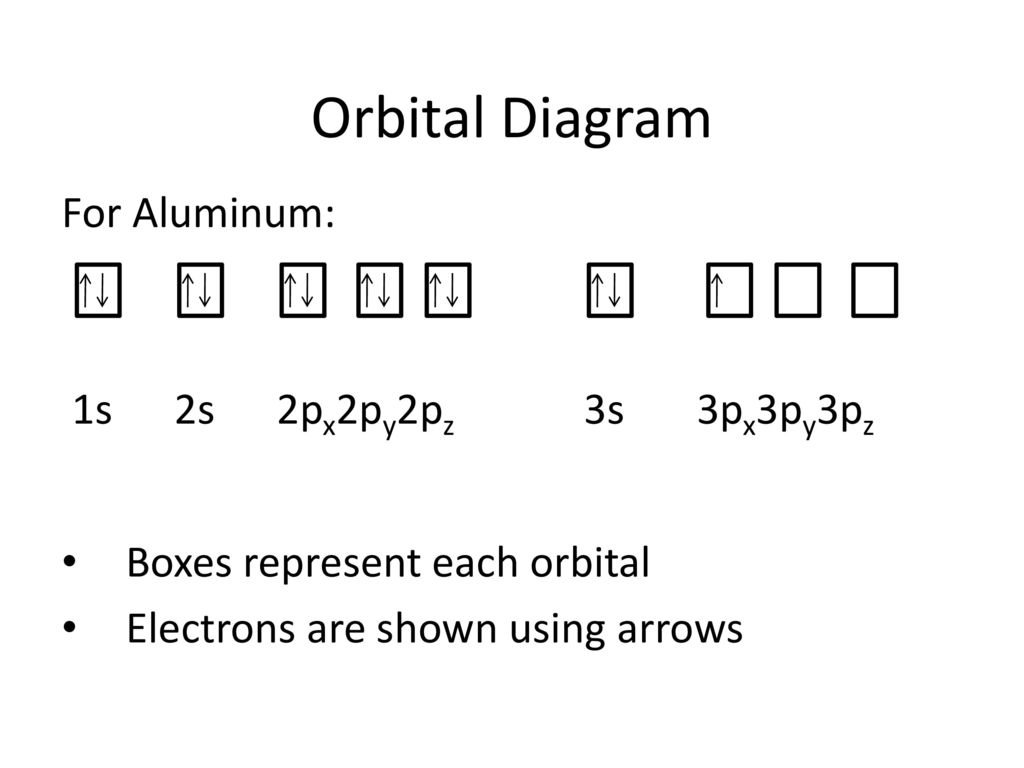
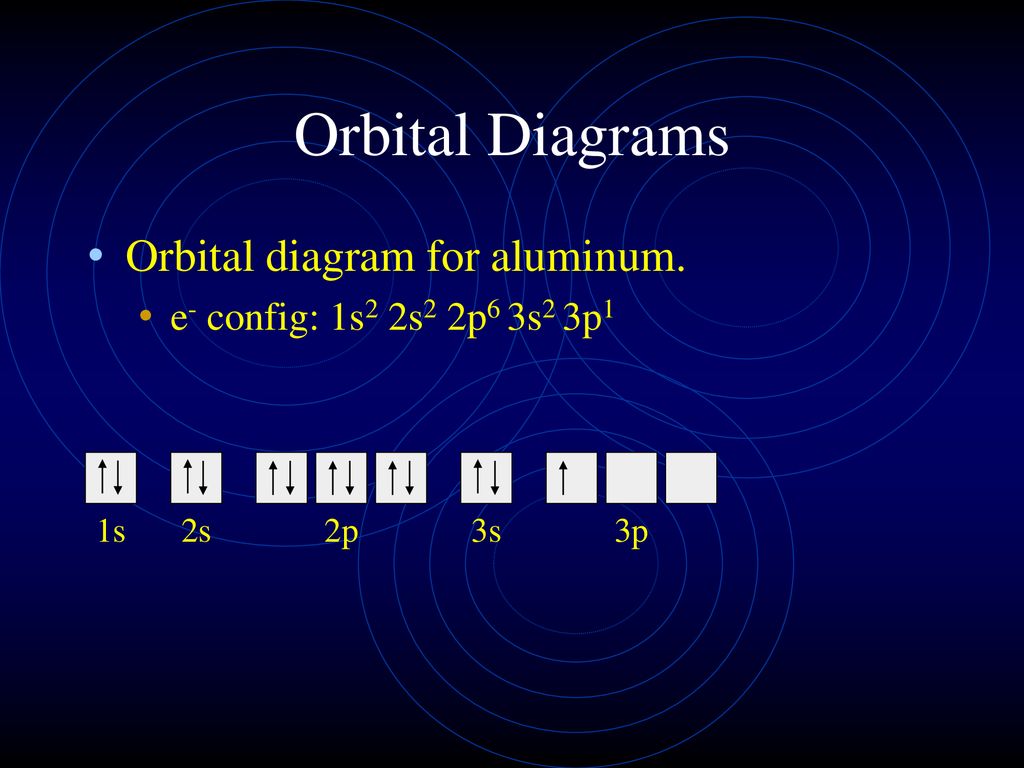
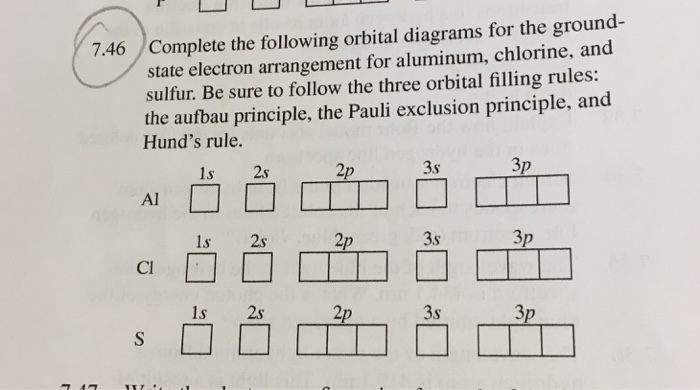
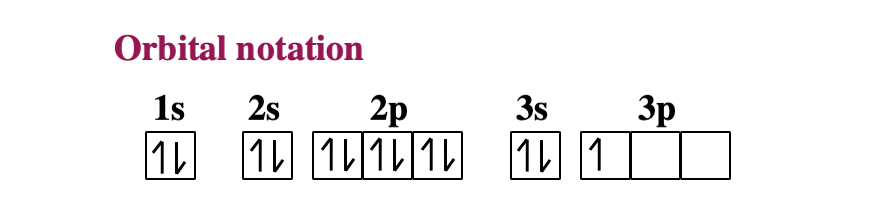
Electron Configuration for Aluminium (Al) Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining electron.
Orbital diagram of aluminum
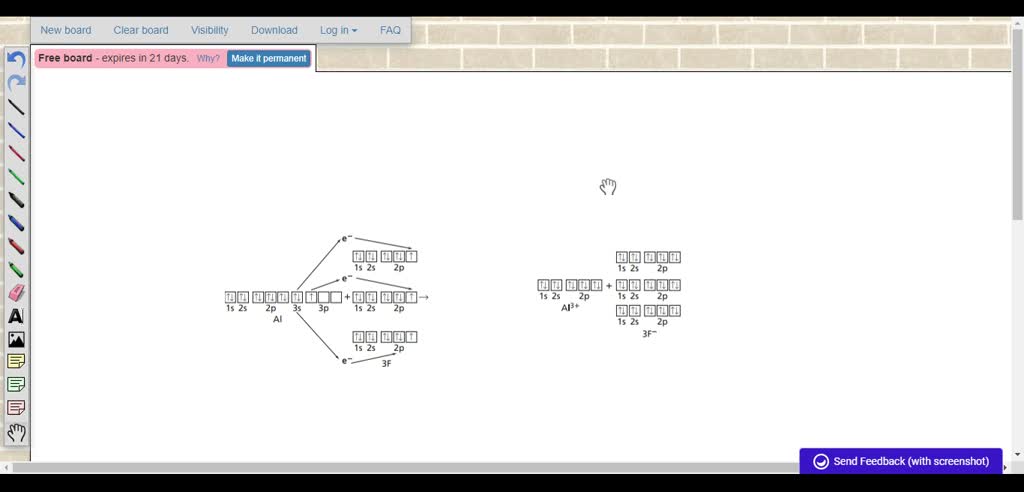
Valence Electron: Definition, Configuration & Example ... 23/09/2021 · Valence electrons, located on an atom's outermost shell, affect how an atom will behave with other atoms. Learn more about a valence electron, including its … orbital notation for aluminum - jamesandjeffreyantiques.com An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum. 1s2 2s2 2p6 3s2 3p1. SOLVED:Using orbital notation, diagram the formation of an ... So in order for aluminum to have a full outer shell, it's gonna lose those the 3/3 energy level electrons of the three orbital electrons. Here is these air aluminum's valence electrons of the ones in the third energy level. So it's gonna give each of those up to Florrie, but each flooring right. If we look at Florence. Orbital notation.
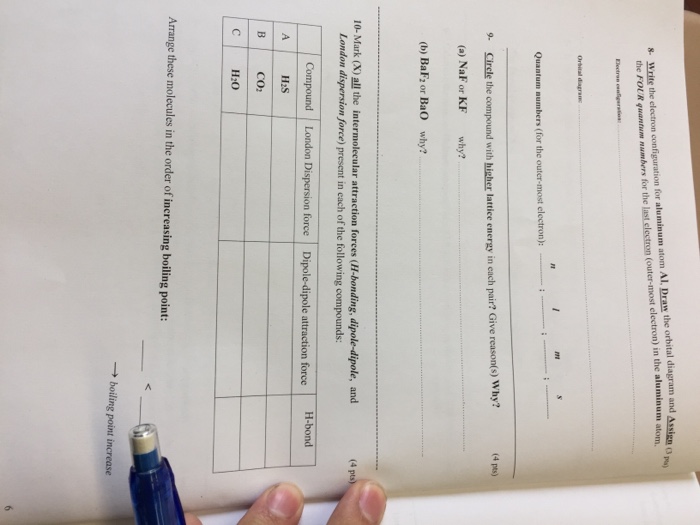
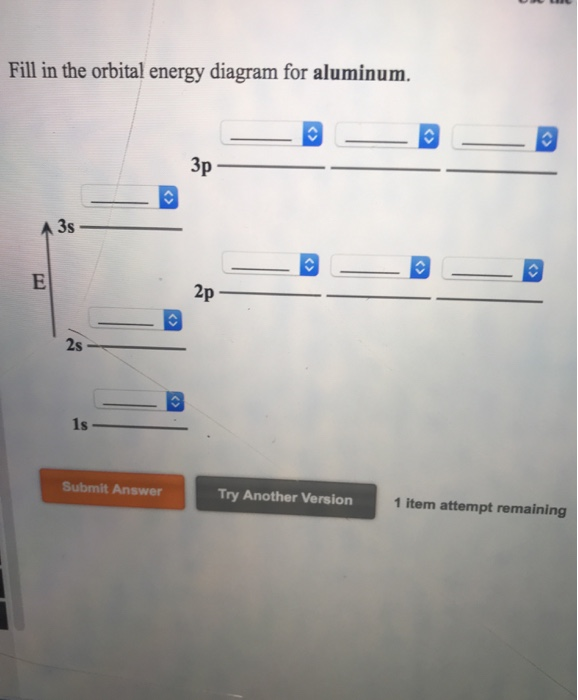
Orbital diagram of aluminum. SCPS Chemistry Worksheet – Periodicity The orbital diagram will show one less e- for each halogen family and each noble gas family with full valence electron shell. A full shell is what the elements strive for so noble gas members require larger IE in order to lose their e-. 7. Where would the largest jump in ionization energies be for oxygen? (with the loss of how many electrons?) The largest ionization jump will occur … Aluminum - Basics The orbital diagram of aluminum helps show the specific "address" of each electron. Each arrow in the diagram represents a single electron (arrow pointing up if positive spin, down if negative spin). The numbers above the squares of the diagram represent the energy level, and the letters represent sub levels. Each box represents an orbital, also. Solved Use the orbital-filling diagram to show the ... Use the orbital-filling diagram to show the electron configuration of aluminum, Al. Be sure to arrange the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. Aluminium/Aluminum (Al) - ChemicalAid Aluminium/Aluminum (Al) has an atomic mass of 13. ... Orbital Diagram. Al - Aluminium/Aluminum - Orbital Diagram - Electron Configuration ...
Physical Properties of Period 3 ... - Chemistry LibreTexts The decrease at aluminum: The value for aluminum might be expected to be greater than that of magnesium due to the extra proton. However, this effect is offset by the fact that the outer electron of aluminum occupies a 3p orbital rather than a 3s orbital. The 3p electron is slightly farther from the nucleus than the 3s electron, and partially screened by the 3s electrons as well as the … 6.4 Electronic Structure of Atoms (Electron Configurations ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... S orbital can hold maximum of 2 electrons and P orbital can hold maximum of 6 electrons. FAQ. How many electron shells a Carbon Bohr model contains? Electron shell also called energy level, you can find the number of electron shells for an element by knowing its period number in the periodic table. The elements or atoms in the first period of the periodic table have one energy … Apply the rules and principles of electron ... - Brainly.com Apply the rules and principles of electron configuration to draw the orbital diagram of aluminum. Use the periodic table to help you. - 14376321 hendersonanj hendersonanj 01/10/2020 Chemistry Middle School answered • expert verified
Answered: II. Directions: Draw the orbital… | bartleby Solution for II. Directions: Draw the orbital diagram of the following elements and identify the type of magnetic property. 11. Aluminum 12. Manganese Silver(Ag) electron configuration and orbital diagram Because the atom may be in a more stable state when the orbital is half-filled and full-filled. Therefore, an electron of 5s orbital completes a full-filled 4d orbital by jumping into a 4d orbital. Therefore, the silver(Ag) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. How to write the orbital diagram for ... Parabolic antenna - Wikipedia A parabolic antenna is an antenna that uses a parabolic reflector, a curved surface with the cross-sectional shape of a parabola, to direct the radio waves.The most common form is shaped like a dish and is popularly called a dish antenna or parabolic dish.The main advantage of a parabolic antenna is that it has high directivity.It functions similarly to a searchlight or … PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
Orbital Diagrams - Concept - Chemistry Video by Brightstorm Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ...
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
(Get Answer) - Part A The Blank Molecular Orbital Diagram ... Molecular Orbital Diagrams and Bond Order Constants Periodic Table Part A The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron carbon, or nitrogen. Bonding orbitals are marked with s...
What is the orbital diagram of aluminum? - Quora Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
Aluminum electron configuration Electronic configuration of the Aluminum atom. Valence electrons. Orbital diagram.
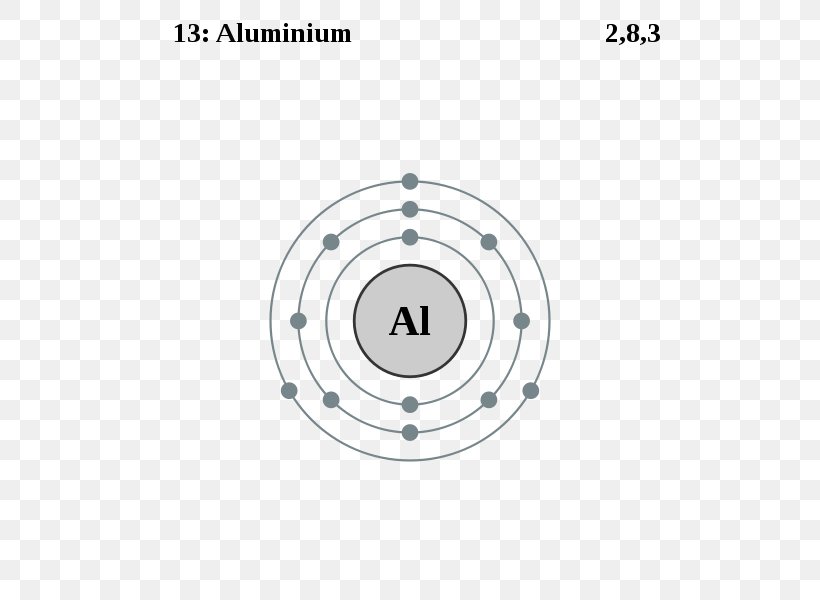
Aluminum, atomic structure - Stock Image - C018/3694 ... Aluminum (Al, US spelling). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of aluminum-27 (atomic number: 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (orange). 13 electrons (white) occupy available electron shells (rings).
Give the orbital diagram for aluminum. | Study.com To determine the electron configuration and draw an orbital diagram of aluminum, we follow 3 rules: Aufbau principle: This states we fill starting from lower energy levels and progress to the next ...
Exceptions to the Octet Rule | Boundless Chemistry Boron and aluminum, from Group III (or 13), display different bonding behavior than previously discussed. These atoms each have three valence electrons, so we would predict that these atoms want to bond covalently in order to gain 5 electrons (through sharing) to fulfill the octet rule. However, compounds in which boron or aluminum atoms form five bonds are never observed, …
Solved Item 15 150 Constants Period Review Use the | Chegg.com Item 15 150 Constants Period Review Use the orbital-filling diagram to show the electron configuration of aluminum, Al. Learning Goal: Relate orbital-filling diagrams to electron configurations. An electron configuration shows the occupation of orbitals by electrons for a particular atom. For example, He has two electrons in the 18 orbital.
Aluminum Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
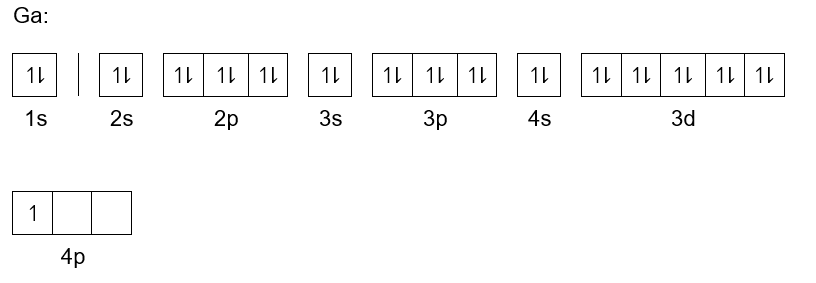
Gallium Orbital Diagram - schematron.org The orbital structure of gallium is simple as in the first eighteen elements. It adds its electron to the outermost shell in the way aluminum and boron do. Comprehensive information for the element Gallium - Ga is provided by this page including scores of properties, element names in many languages, most known nuclides and .
What is the orbital diagram for aluminum? - Answers Oct 19, 2009 · An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron.
Orbital Diagram For Xenon - schematron.org An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum.
Al2O3 Lewis Structure, Molecular Geometry, Hybridization ... The hybrid orbital formed by mixing of one s and two p-orbital forms trigonal symmetry and is maintained at an angle of 120°. Polarity of Al2O3 A condition in which two opposite charges viz. positive and negative are located on the same molecule or atom is known as polarity.
Magellan (spacecraft) - Wikipedia The Magellan spacecraft was a 1,035-kilogram (2,282 lb) robotic space probe launched by NASA of the United States, on May 4, 1989, to map the surface of Venus by using synthetic-aperture radar and to measure the planetary gravitational field.. The Magellan probe was the first interplanetary mission to be launched from the Space Shuttle, the first one to use the Inertial …
Aluminum(Al) electron configuration with orbital diagram Orbital diagram for aluminum(Al) The next three electrons will enter the 2p orbital in the clockwise direction and the next three electrons will enter the 2p orbital in the anti-clockwise direction. The next two electrons will enter the 3s orbital and the remaining one electron will enter the 3p orbital in a clockwise direction.
Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
Phosphorus(P) electron configuration and orbital diagram Orbital diagram for phosphorus (P) Phosphorus(P) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub ...
SOLVED:Using orbital notation, diagram the formation of an ... So in order for aluminum to have a full outer shell, it's gonna lose those the 3/3 energy level electrons of the three orbital electrons. Here is these air aluminum's valence electrons of the ones in the third energy level. So it's gonna give each of those up to Florrie, but each flooring right. If we look at Florence. Orbital notation.
orbital notation for aluminum - jamesandjeffreyantiques.com An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum. 1s2 2s2 2p6 3s2 3p1.
Valence Electron: Definition, Configuration & Example ... 23/09/2021 · Valence electrons, located on an atom's outermost shell, affect how an atom will behave with other atoms. Learn more about a valence electron, including its …




















0 Response to "39 Orbital Diagram Of Aluminum"
Post a Comment