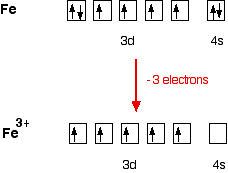
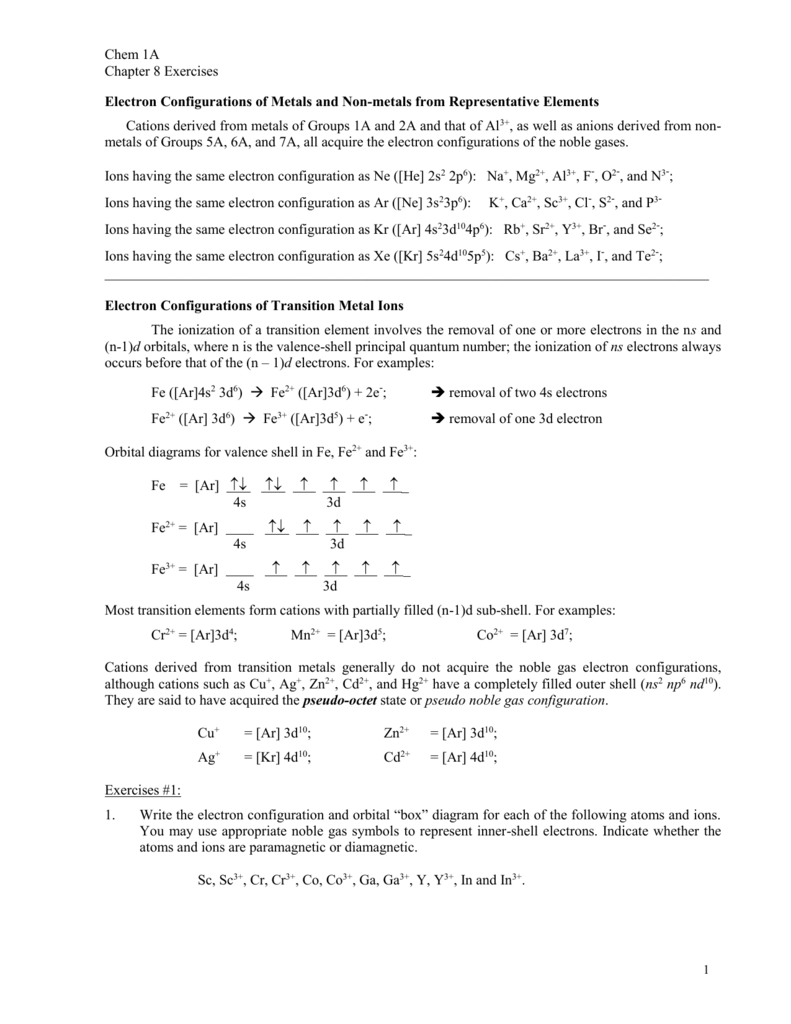
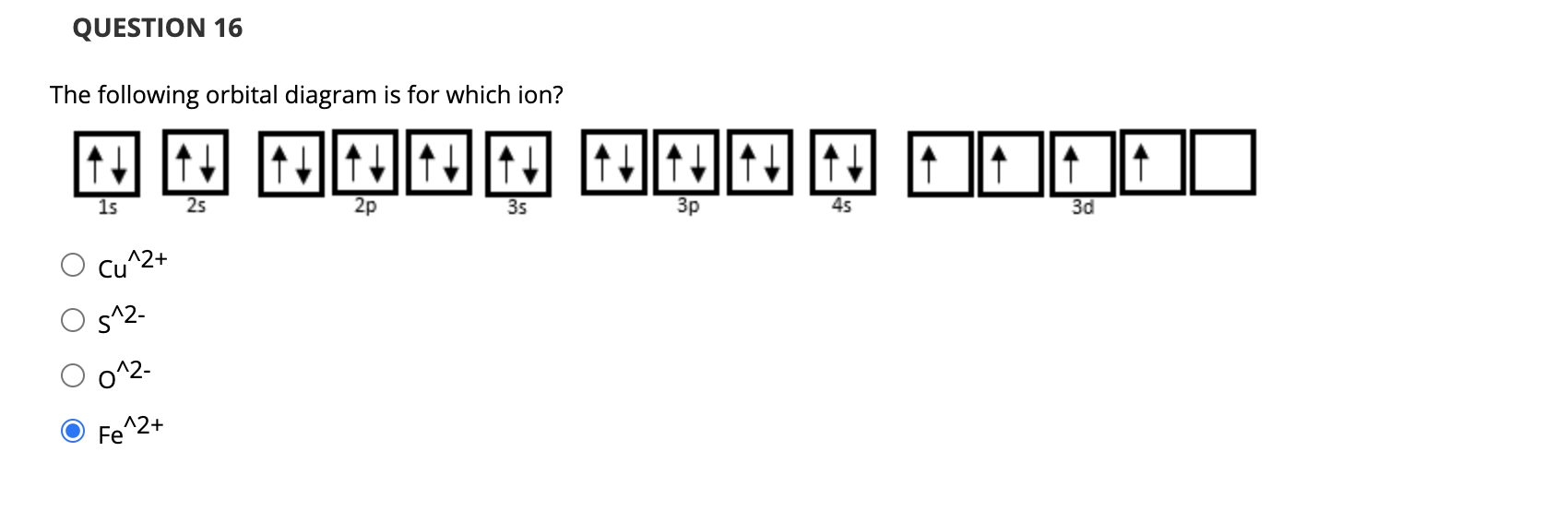
40 Fe 2+ Orbital Diagram
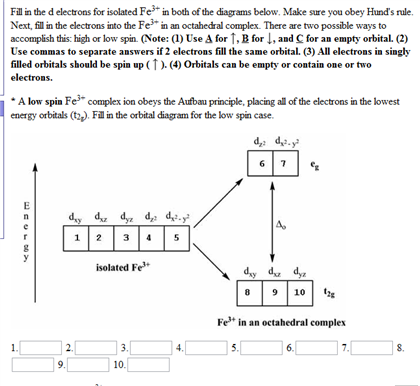
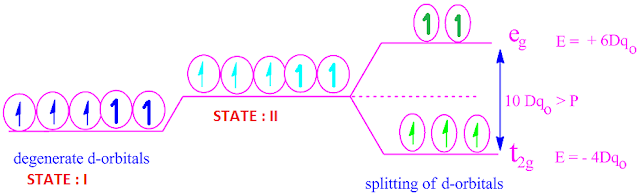
Tanabe–Sugano diagram - Wikipedia Unnecessary diagrams: d 1, d 9 and d 10 d 1. There is no electron repulsion in a d 1 complex, and the single electron resides in the t 2g orbital ground state. A d 1 octahedral metal complex, such as [Ti(H 2 O) 6] 3+, shows a single absorption band in a UV-vis experiment. The term symbol for d 1 is 2 D, which splits into the 2 T 2g and 2 E g states. The t 2g orbital set holds the single ... Small-Body Database Lookup - NASA Instructions. The search form recognizes IAU numbers, designations, names, and JPL SPK-ID numbers. When searching for a particular asteroid or comet, it is best to use either the IAU number, as in 433 for asteroid “433 Eros”, or the primary designation as in 1998 SF36 for asteroid “25143 (1998 SF36)”.However, using the asteroid/comet name will also work, as in Ceres for …
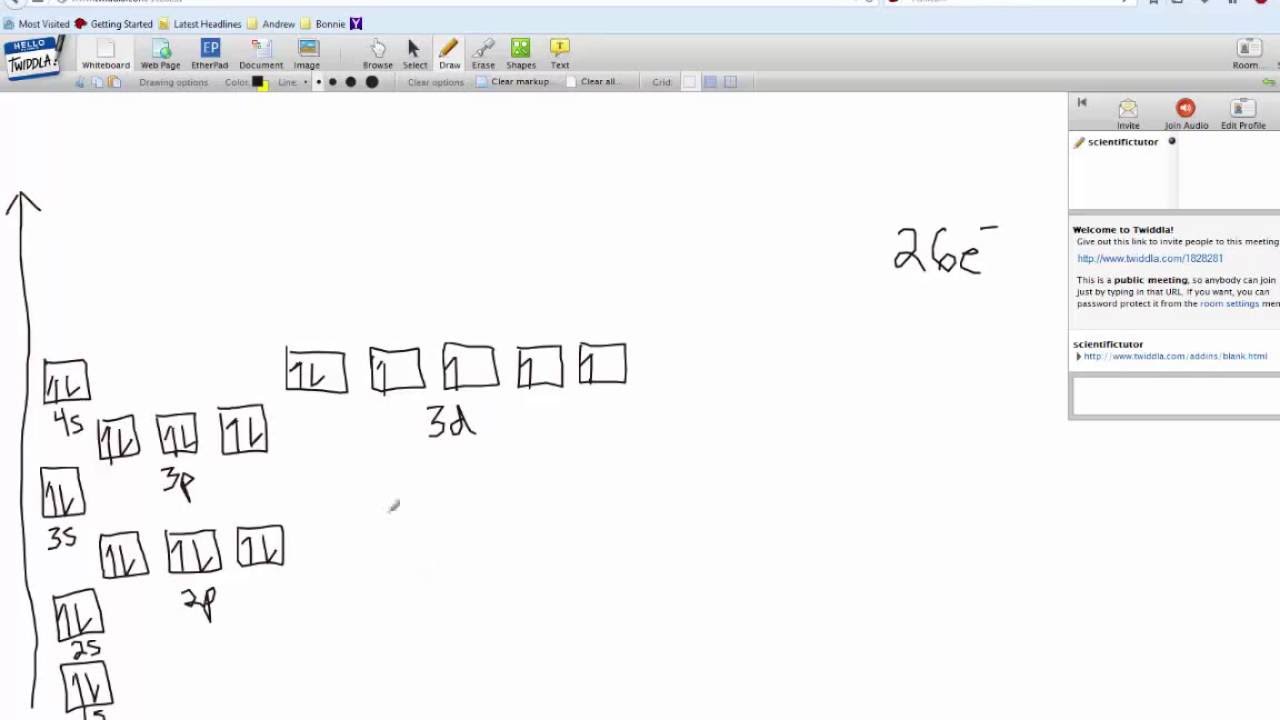
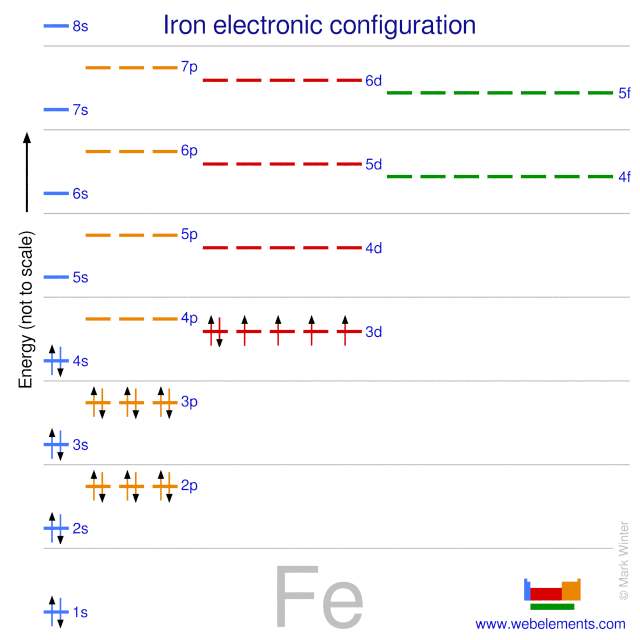
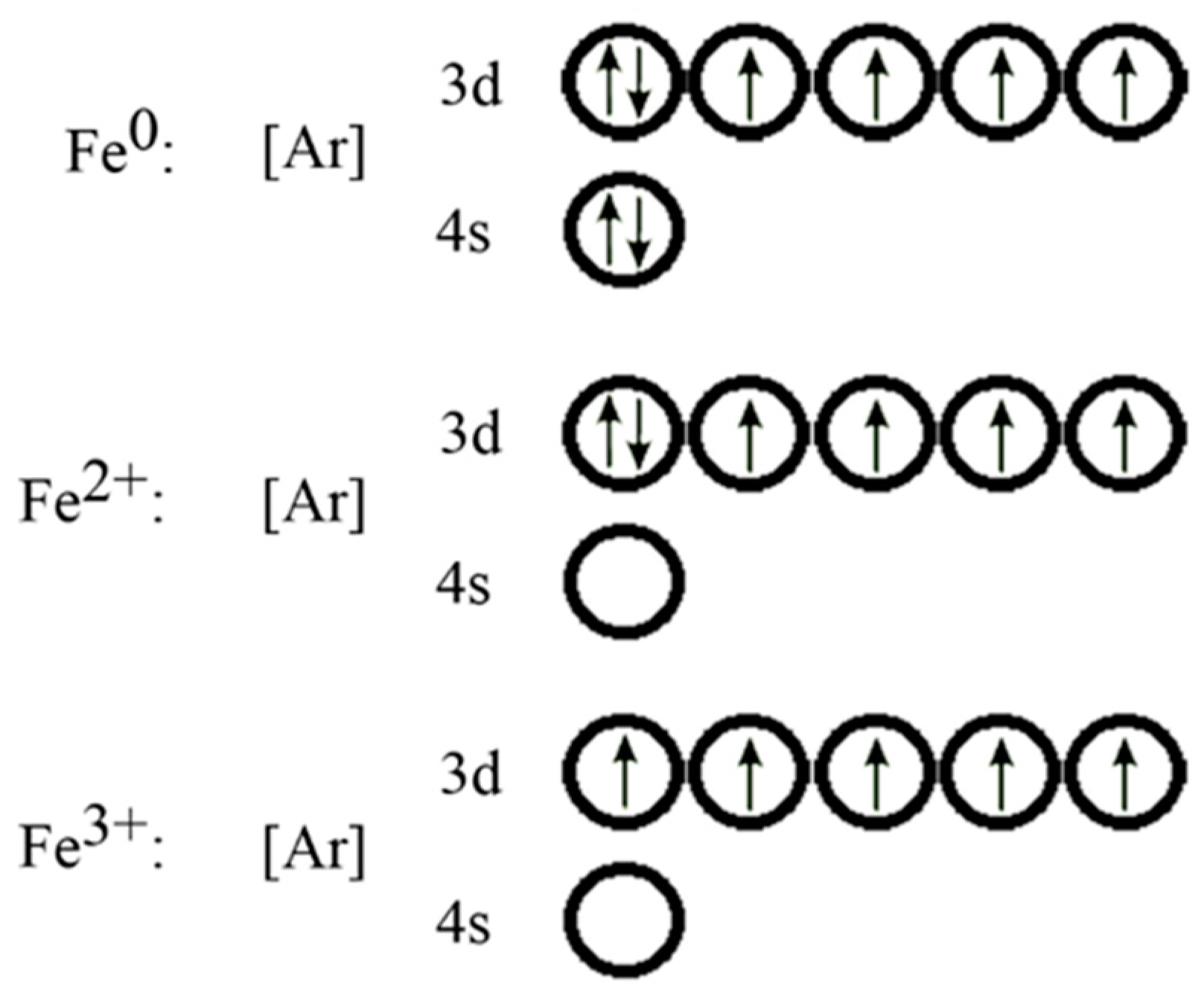
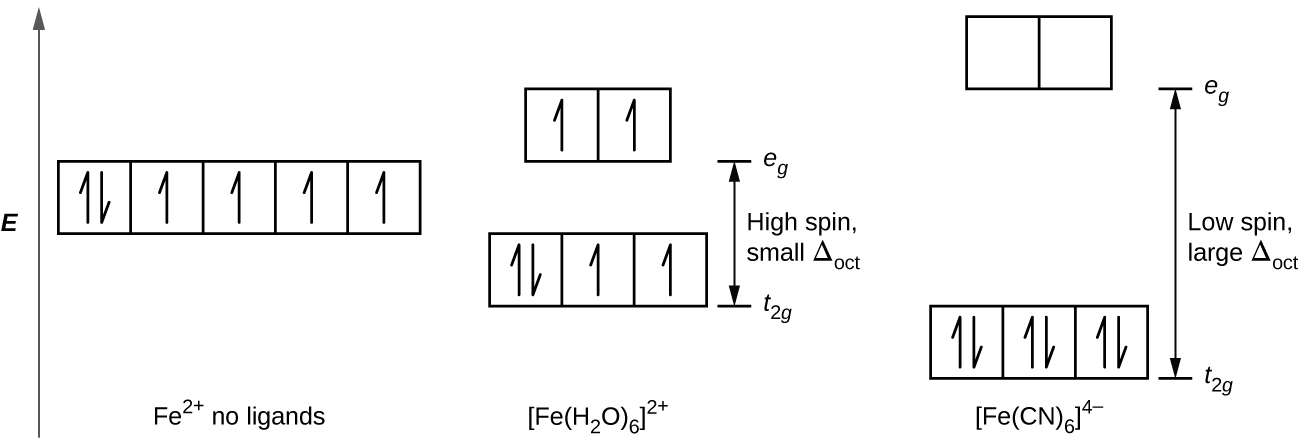
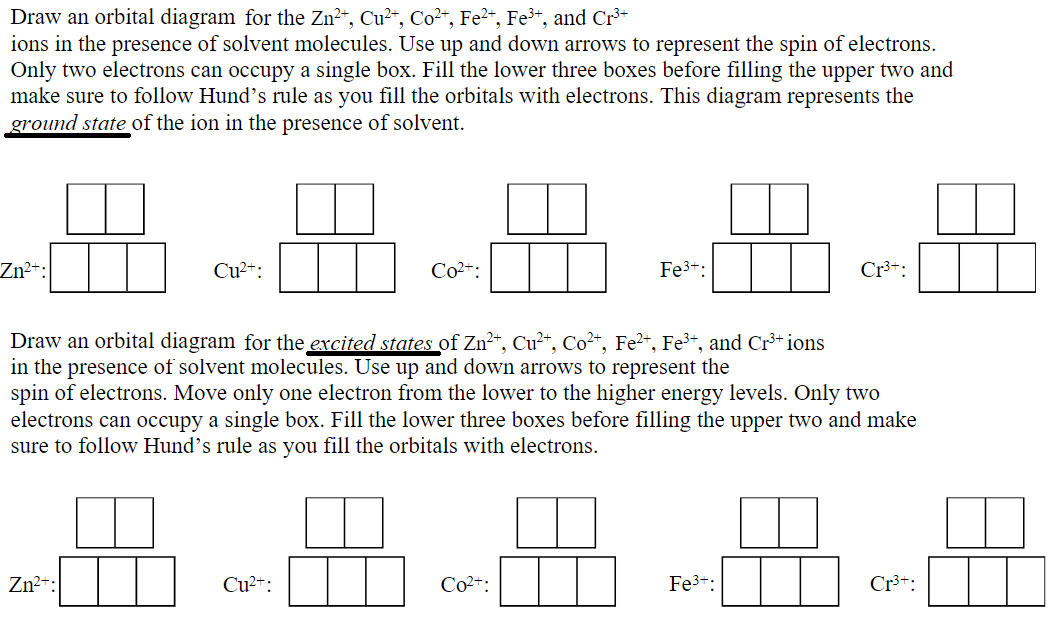
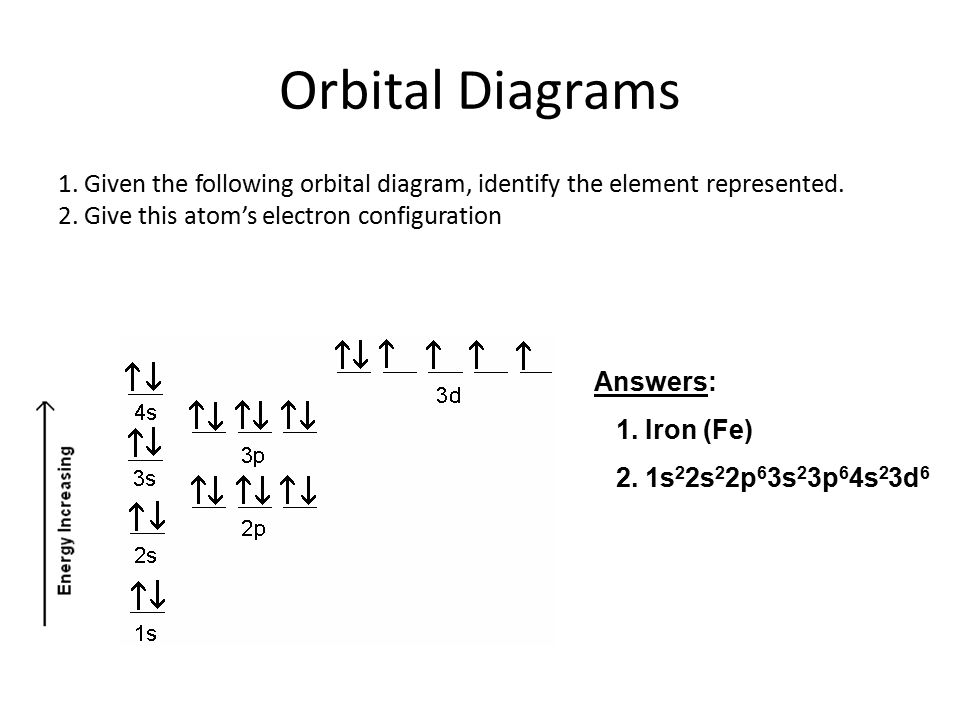
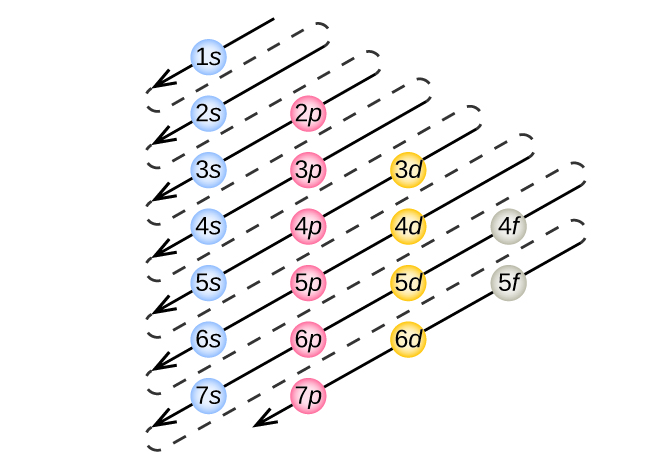
Quantum Numbers, Atomic Orbitals, and Electron Configurations Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square (or circle), and the electrons as arrows pointing up or down (indicating the electron spin). ... Fe 2+ 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6: Fe 3+

Fe 2+ orbital diagram
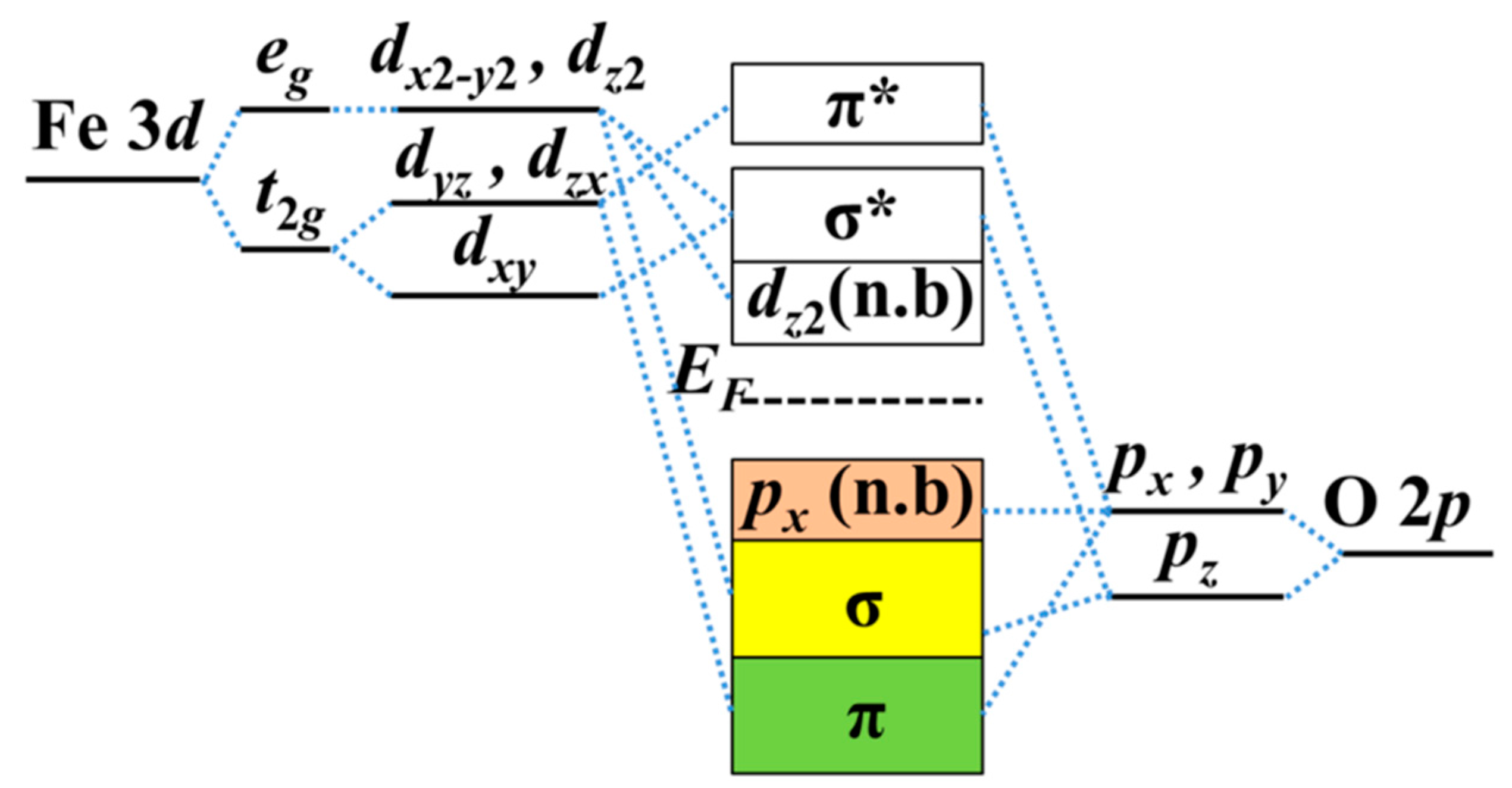
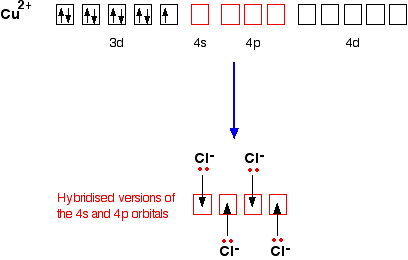
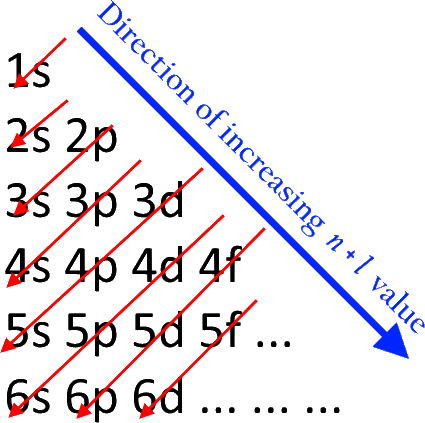
Quantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. The Organometallic HyperTextBook: Cyclopentadienyl Ligands Mar 31, 2015 · The first characterized example of a cyclopentadienyl complex was ferrocene, Cp 2 Fe, which has an iron atom "sandwiched" between two planar Cp rings as shown on the left. ... a molecular orbital (MO) diagram best describes the bonding in a Cp complex. Consider the five MO's of a Cp ligand. If we have two of these, we can add and subtract ... Insights into mechanism of Fe-dominated active sites via ... Calculated band structure, DOS and corresponding LUMO and HOMO distribution of (a,d) CN-Fe-P, (b,e) CN-Ni-P, (c,f) CN-FeNi-P, (g) Free energy diagram of the samples for O 2 –, * means the catalytic sites, (h) Schematic diagram of electron transfer in CN-FeNi-P, (i) Difference charge density profile for ENO adsorbed on the CN-FeNi-P, yellow ...
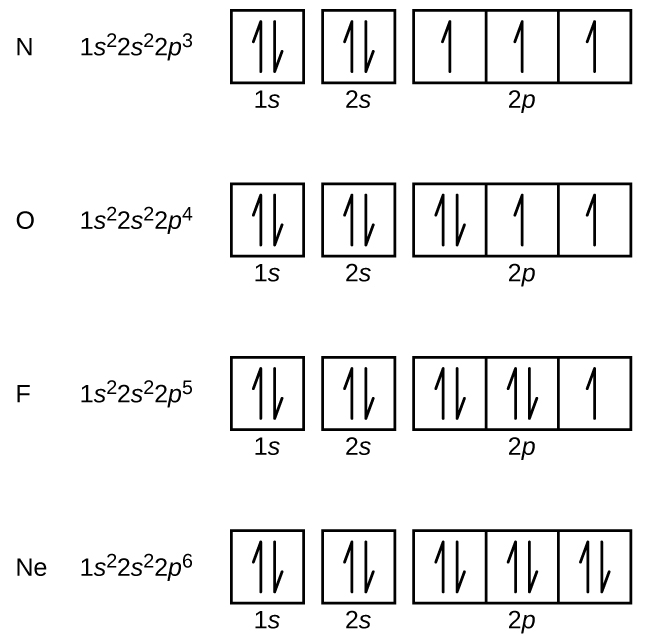
Fe 2+ orbital diagram. COHP - Crystal Orbital Hamilton Population • Theory Theory: From H 2 to Data-Storage Alloys The COHP (or COOP) concept is most easily understood by looking at the simple band structure of a "one-dimensional" solid; the following example has been stolen from a classic introduction.Imagine a linear chain of hydrogen atoms, the one-dimensionally periodic analogue of H 2 (whose molecular-orbital scheme is known … d electron count - Wikipedia The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination … 2 Mineral Chemistry – Mineralogy - OpenGeology The binary (2-component) diagram below (Figure 2.21) shows the compositions plotted on a line anchored by pure forsterite on the left, and pure fayalite on the right. The sample from Burma is nearly 100% forsterite (Mg 2 SiO 4), while that from Germany is 100% fayalite (Fe 2 SiO 4). The other olivines fall between. 2.21 Comparing olivine ... Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ...
Insights into mechanism of Fe-dominated active sites via ... Calculated band structure, DOS and corresponding LUMO and HOMO distribution of (a,d) CN-Fe-P, (b,e) CN-Ni-P, (c,f) CN-FeNi-P, (g) Free energy diagram of the samples for O 2 –, * means the catalytic sites, (h) Schematic diagram of electron transfer in CN-FeNi-P, (i) Difference charge density profile for ENO adsorbed on the CN-FeNi-P, yellow ... The Organometallic HyperTextBook: Cyclopentadienyl Ligands Mar 31, 2015 · The first characterized example of a cyclopentadienyl complex was ferrocene, Cp 2 Fe, which has an iron atom "sandwiched" between two planar Cp rings as shown on the left. ... a molecular orbital (MO) diagram best describes the bonding in a Cp complex. Consider the five MO's of a Cp ligand. If we have two of these, we can add and subtract ... Quantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.



















0 Response to "40 Fe 2+ Orbital Diagram"
Post a Comment