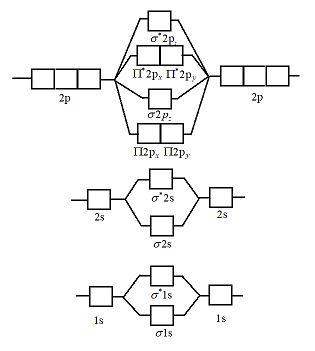
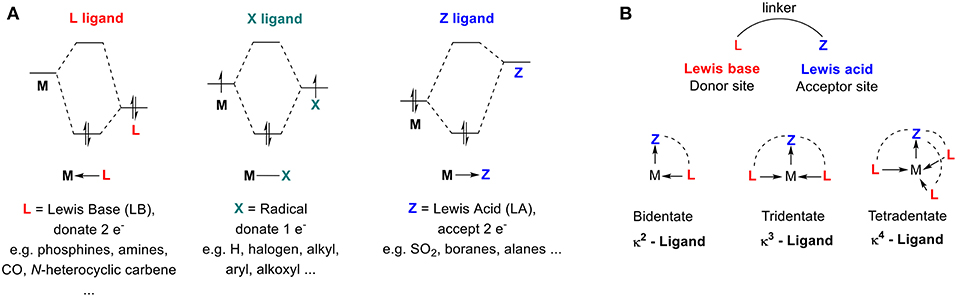
40 li2+ molecular orbital diagram
Which of the following diatomic species are paramagnetic ... A blank molecular orbital diagram (Part B 1 figure) has been provided to help you. Drag the formulas to the appropriate magnetic bin :C2^2+,Li2-,B2^2- 6,762 results Why "Li"_2^+ is more stable than "Li"_2 ... - Socratic.org Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and
Is li2 - paramagnetic or diamagnetic? - TreeHozz.com 4.9/5 (1,377 Views . 33 Votes) A molecular structure that results in unpaired electrons in degenerate MOs, like O2, is called paramagnetic. Li2 has all of its electrons neatly paired up in the orbitals. Li2 is diamagnetic. All this is further explained here.
Li2+ molecular orbital diagram
Molecular orbital theory - W3schools Thus, the electronic configuration of molecular orbital of Li2 molecule is σ1s2 σ*1s2 σ2s2 = KK σ2s2. Since the innermost shell of filled molecular orbitals- σ1s and σ *1s don't make their contribution to the bonding and is occasionally depicted as KK which shows that K-shell is filled completely. Li2 Bond Order - introduction to mot, molecular orbital ... solved given the molecular orbital diagram for dilithium. Li2 Bond Order. Here are a number of highest rated Li2 Bond Order pictures upon internet. We identified it from trustworthy source. Its submitted by giving out in the best field. We agree to this nice of Li2 Bond Order graphic could possibly be the most trending topic following we ... Solved Consider the Molecular Orbital Interaction Diagram ... Transcribed image text: Consider the Molecular Orbital Interaction Diagram for Li2. Which of the following pictures shows the atomic orbitals on the separate atoms that combine to make the LUMO in Liz? The shading represents the phases of the wavefunction. o S + S o S + S o p + P o 88 88 p + p O p + p o р + р Consider the Molecular Orbital Interaction Diagram for N2.
Li2+ molecular orbital diagram. (Solved) - molecular orbital diagram. li2 is stable but ... Nb= Number of electrons in bonding orbitals Na= Number of electrons in anti-bonding orbitals According to Molecular Orbital Theory If Nb > Na, the molecule is stable If Nb=Na, the molecule is unstable If Nb Molecular Orbital Diagram (MO Diagram) of Li2 - YouTube Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out: PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
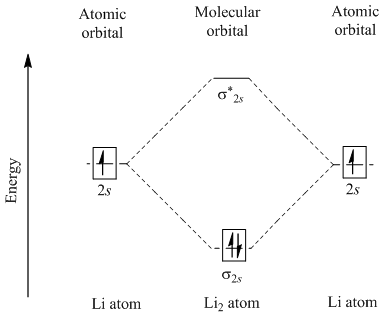
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... Molecular Orbital Diagram For Li2 - schematron.org Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Li2 Molecular Orbital Diagram Jul 02, 2019 · Molecular orbital energy level of Li2.This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as . Write the electronic configuration of Lithium (Li2 ... Reason The number of electrons in antibonding molecular orbitals is two less than in bonding molecule orbitals.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Li2 Molecular orbital diagram - Brainly.in Nov 01, 2018 · Li2 Molecular orbital diagram - 6473509 Which of the following is paramagnetic? (use the mole... Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos. IIT JEE - Bonding in Homonuclear Diatomic Molecules: Li2 ... Get access to the latest Bonding in Homonuclear Diatomic Molecules: Li2, Li2+, Be2, B2, C2, N2 prepared with IIT JEE course curated by Megha Khandelwal on Unacademy to prepare for the toughest competitive exam.
How to predict the existence of Li2 C2 with their ... Answer: This is a very ambitious calculation. F. A. Matsen did such a calculation on the molecule Li-H about fifty years ago, but the calculations have become more streamlined since then. The results from such calculations will not actually predict the "existence" of Li2C2, but will provide a re...
3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
Use the molecular orbital diagram shown to determine which ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
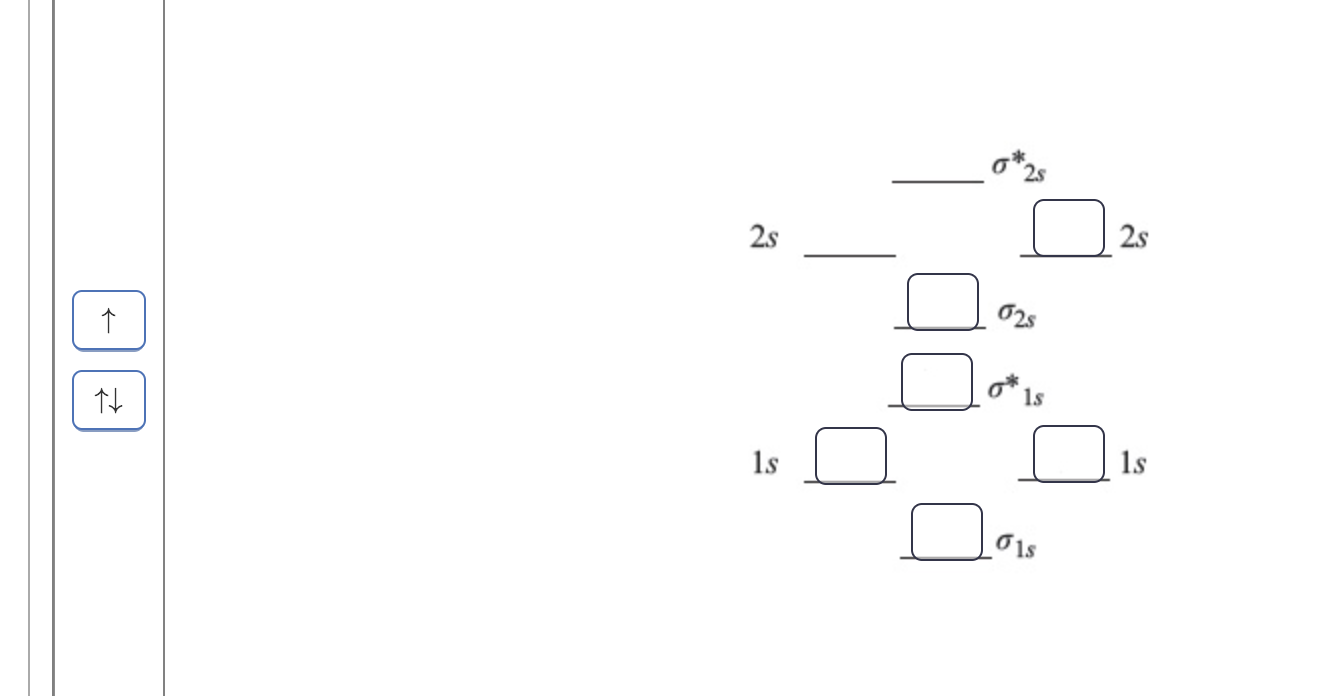
Solved Construct the molecular orbital diagram for Li2 ... Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5. Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown.
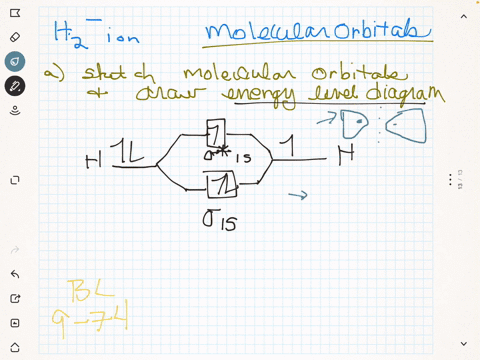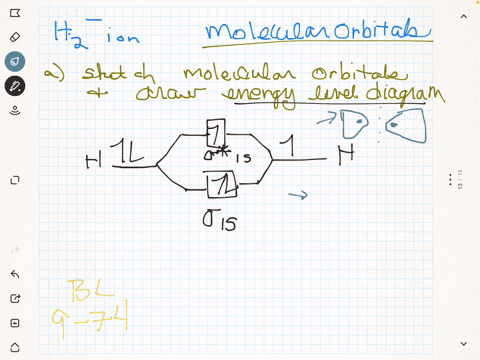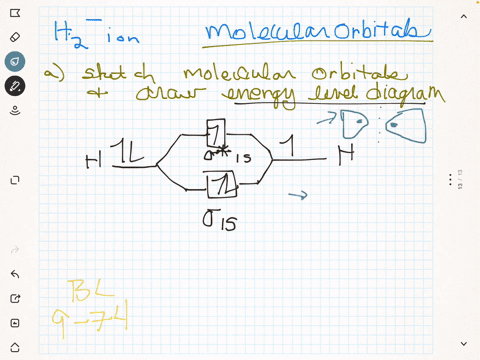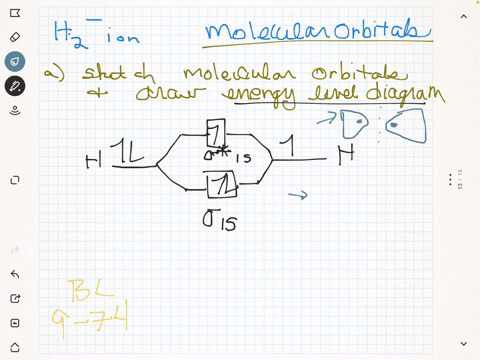
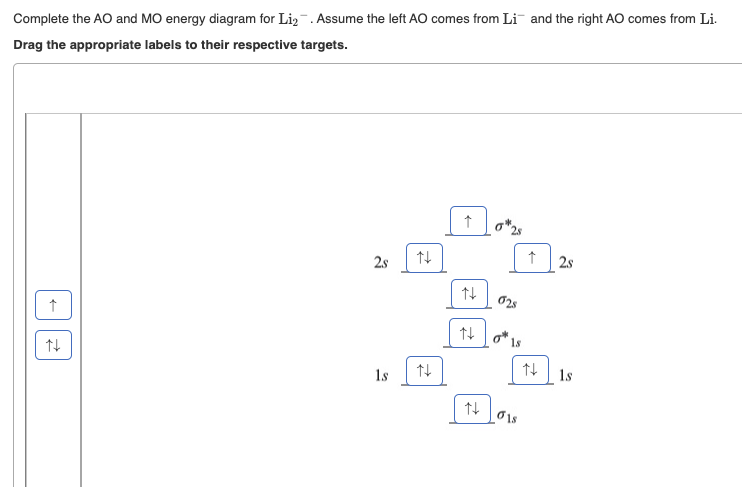
(Get Answer) - Draw a simple molecular orbital diagram for ... 1. Draw an Molecular Orbital energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules to exist in the gas phase?( Li2+: 2 is subscript, + is superscript)2. Draw an Molecular Orbital energy diagram and predict the bond...
MOT | Molecular Orbital Energy level Diagram for Li2, Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o...
Li2- Molecular Orbital Diagram - schematron.org Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Answer to Draw a molecular ...
Molecular Orbitals of Li₂ to F₂ - Chemistry LibreTexts The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to other diatomic molecules, but involving more than the 1s atomic orbitals. For the second period elements, the 2s and 2p orbitals are important for MO considerations. A linear combination of properly oriented atomic orbitals for the formation of sigma s and pi p bonds.
Molecular Orbital Diagram For Li2 - Wiring Diagrams Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
Li2- Molecular Orbital Diagram Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
Molecular Orbital Theory |Molecular Orbital Theory Bangla ... Molecular Orbital Theory |Molecular Orbital Theory Bangla | Bond Order |Bond Length| Li2|molecular orbital theory,molecular orbital diagram,molecular orbital...
Molecular Orbitals - Introductory Chemistry - 1st Canadian ... The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
Solved Consider the Molecular Orbital Interaction Diagram ... Transcribed image text: Consider the Molecular Orbital Interaction Diagram for Li2. Which of the following pictures shows the atomic orbitals on the separate atoms that combine to make the LUMO in Liz? The shading represents the phases of the wavefunction. o S + S o S + S o p + P o 88 88 p + p O p + p o р + р Consider the Molecular Orbital Interaction Diagram for N2.
Li2 Bond Order - introduction to mot, molecular orbital ... solved given the molecular orbital diagram for dilithium. Li2 Bond Order. Here are a number of highest rated Li2 Bond Order pictures upon internet. We identified it from trustworthy source. Its submitted by giving out in the best field. We agree to this nice of Li2 Bond Order graphic could possibly be the most trending topic following we ...
Molecular orbital theory - W3schools Thus, the electronic configuration of molecular orbital of Li2 molecule is σ1s2 σ*1s2 σ2s2 = KK σ2s2. Since the innermost shell of filled molecular orbitals- σ1s and σ *1s don't make their contribution to the bonding and is occasionally depicted as KK which shows that K-shell is filled completely.









![Solved] Using Figures 9.35 and 9.43 as guides, draw the ...](https://s3.amazonaws.com/si.question.images/image/images11/876-(557)-2.png)








0 Response to "40 li2+ molecular orbital diagram"
Post a Comment