40 Use The Orbital-filling Diagram To Show The Electron Configuration Of Helium, He.
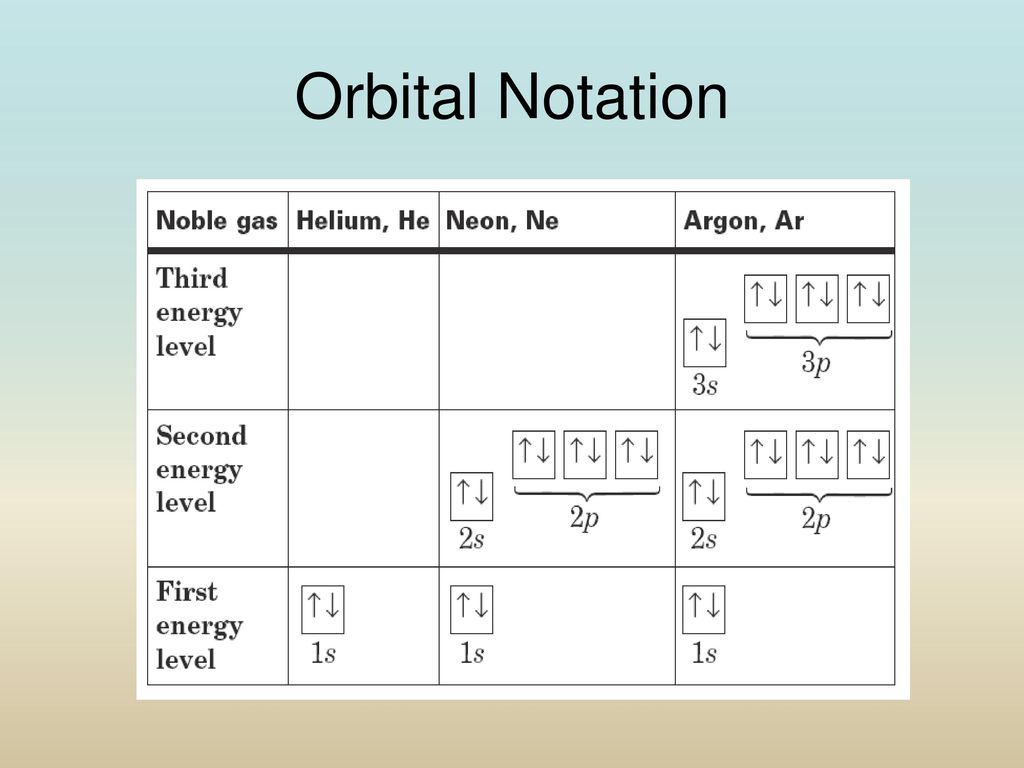
Electron Configuration Practice Worksheet - SharpSchool 2. Electron Configuration (quicker to draw than orbital filling diagrams) Ex. O2 1s2 2s2 2p4. 3. Electron Dot shows only the valence (outer energy level) electrons. . Ex. Oxygen atom . O :. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram Electron Configuration for Phosphorus (P) - UMD The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3.
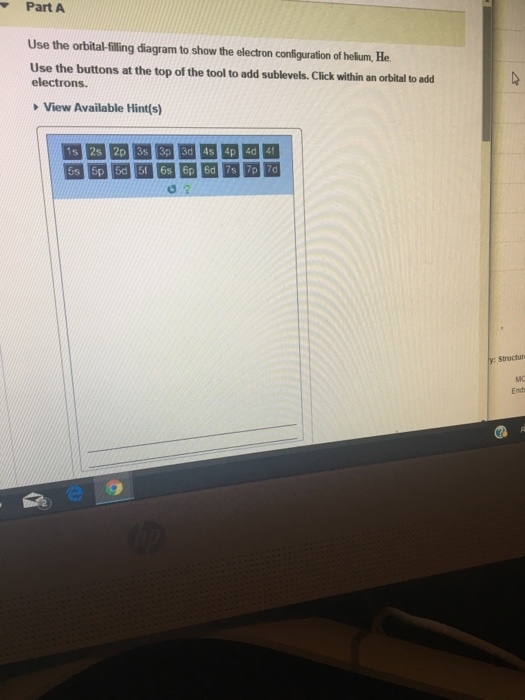
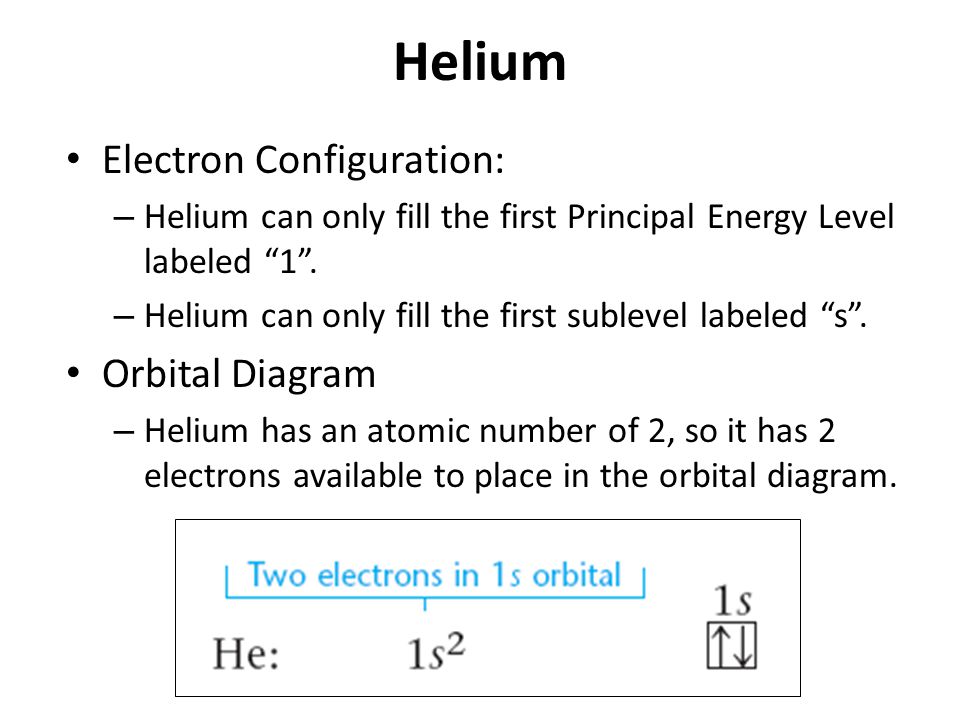
Use the orbital-filling diagram to show th... | Clutch Prep Problem Details. Use the orbital-filling diagram to show the electron configuration of helium, He. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. Learn this topic by watching The Electron Configuration Concept Videos.

Use the orbital-filling diagram to show the electron configuration of helium, he.
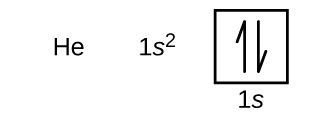
Neon Orbital diagram, Electron configuration, and Valence ... The ground-state electron configuration of the Neon (Ne) atom is 1s22s22p6. And for the excited state, it is 1s 2 2s 2 2p 5 3s 1. The shorthand electron configuration for Neon is [He] 2s 2 2p 6. The number of valence electrons available for Neon atoms is 8. Neon is situated in Group 18th and has an atomic number of 10. Solved Part A Use the orbital-filling diagram to show the ... Chemistry questions and answers. Part A Use the orbital-filling diagram to show the electron configuration of helium, He. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. View Available Hint (s) 15 23 22 35 3p 3d 4s 4p 4d 4 55 5P 5d 5f 6s 6p 6d 75 7P 7d Part B Use the orbital-filling diagram to ... Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital.
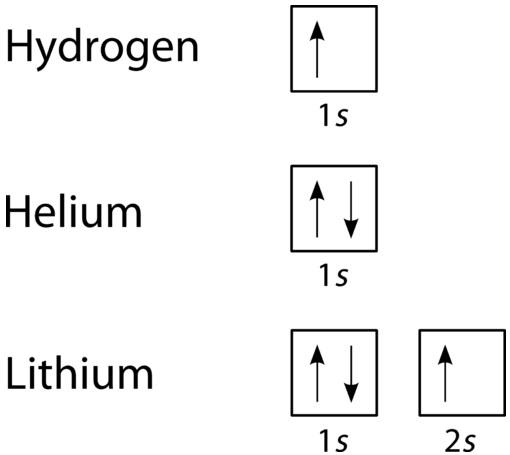
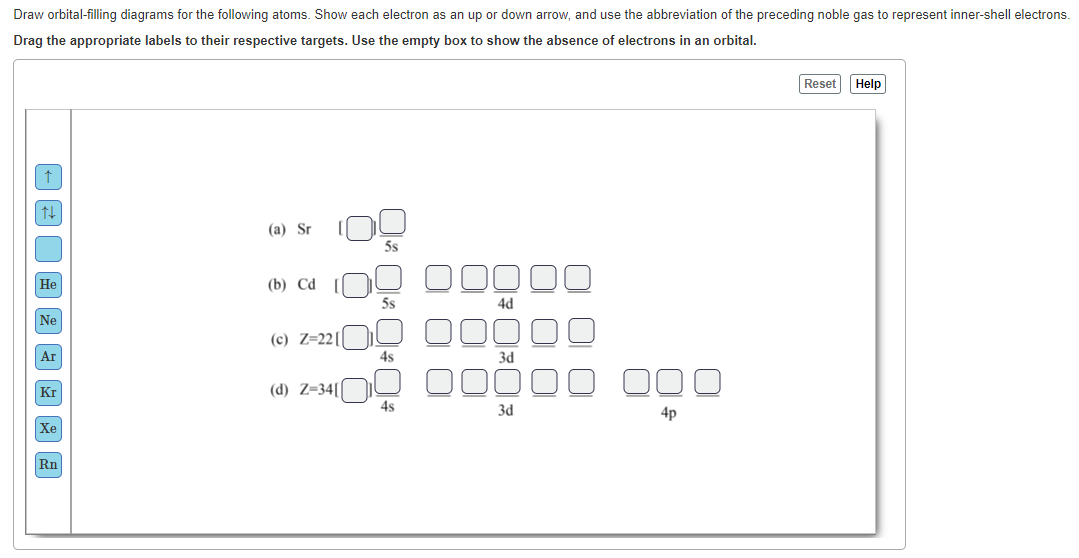
Use the orbital-filling diagram to show the electron configuration of helium, he.. Solved Use the orbital-filling diagram to show the ... Use the orbital-filling diagram to show the electron configuration of helium, He. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled. What is the electron configuration for helium? | Socratic This means that the electron configuration for helium has to account for only 2 electrons. Both of these electrons are located on the first energy level, in the only subshell, and consequently only orbital available to them. More specifically, both electrons will occupy the 1s orbital, the only orbital located in the s subshell. Ruth Montag Chapter 8 Mastering Chemistry - Quizlet Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
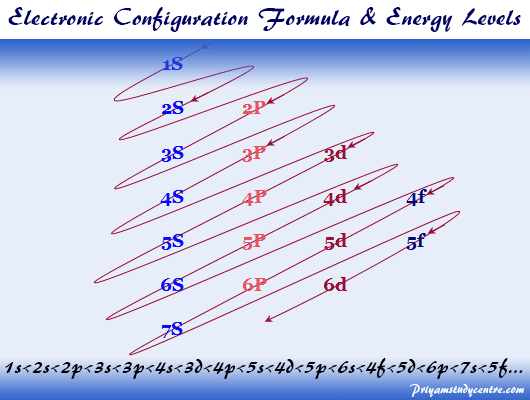
Solved Electron Configurations Use the orbital-filling ... Expert Answer Transcribed image text: Electron Configurations Use the orbital-filling diagram to show the electron configuration of helium, He. Drag the appropriate labels to their respective targets. Labels can be used once more than once, or not at all. Not all targets will be filled. View Available Hint (s) Reset Help Electron Configuration for Neon (Ne) - UMD How to Write the Electron Configuration for Neon. Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. PDF Electron Config & Orbital Filling Answer Key Electron Arrangements Name There are three ways to indicate the arrangement of electrons around an atom: 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. 1.8: Filling Orbitals with Electrons - Chemistry LibreTexts One of the shortcuts that is often used when writing electron configuration is to show "core" electrons simply as the inert gas from the preceding period. For example, fluorine is in the second period (n = 2). That means that the orbitals associated with the first period are already filled, just like they are in the inert gas, helium (He).
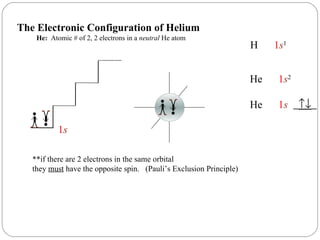
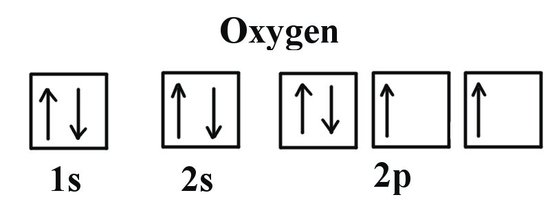
Bohr Model, Electron Config, and Orbital Diagrams - Quizlet Start studying Bohr Model, Electron Config, and Orbital Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Electron Configuration for Helium (He) Helium only has 2 electrons and therefore it has a configuration of 1s 2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. This means it will not react with other atoms. Solved Part B Use the orbital-filling diagram to show the ... Transcribed image text: Part B Use the orbital-filling diagram to show the electron configuration of oxygen, O. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled. View Available Hint(s) Reset Help 1 11 1s 2s 3s 2p 3p G1 G1 G1 || G1G1 G1 G1G11 G1 G2 G2 G2 G2 G2 Electron Configurations Part C Use the ... How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
Chemistry Chapter 5 Electromagnetic Radiation Flashcards ... Use the orbital-filling diagram to show the electron configuration of helium, He. Use the orbital-filling diagram to show the electron configuration of oxygen, O. Use the orbital-filling diagram to show the electron configuration of gallium, Ga.
Chapter 8 Mastering Chemistry Flashcards - Quizlet Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As).
Electron Configuration for Aluminium (Al) The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining electron. Therefore the Aluminium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 1.
Solved Part A Use the orbital-filing diagram to show the ... Chemistry Chemistry questions and answers Part A Use the orbital-filing diagram to show the electron configuration of helium, He. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled.
What is the full orbital diagram of He? - Answers Definition of orbital diagram? An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and...
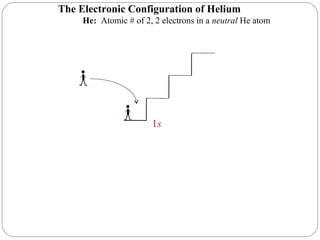
Arrangements of electrons in the orbitals of an atom is ... The orbital diagram for helium is, So while hydrogen has the electron configuration of 1s 1 , helium has the electron configuration of 1s 2. The energy diagram for helium is shown as here. Notice that there has been a change in the relative energies of the 2s and 2p orbitals. This is an important point that must be addressed at this point.
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Atomic Structure: Electron Configuration and Valence ... For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic.
MULUI3627beabcb0ad8867dcf2d783aa0c#10001 Text Use the ... Question: MULUI3627beabcb0ad8867dcf2d783aa0c#10001 Text Use the orbital-filling diagram to show the electron configuration of helium, He.
Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital.
Solved Part A Use the orbital-filling diagram to show the ... Chemistry questions and answers. Part A Use the orbital-filling diagram to show the electron configuration of helium, He. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. View Available Hint (s) 15 23 22 35 3p 3d 4s 4p 4d 4 55 5P 5d 5f 6s 6p 6d 75 7P 7d Part B Use the orbital-filling diagram to ...
Neon Orbital diagram, Electron configuration, and Valence ... The ground-state electron configuration of the Neon (Ne) atom is 1s22s22p6. And for the excited state, it is 1s 2 2s 2 2p 5 3s 1. The shorthand electron configuration for Neon is [He] 2s 2 2p 6. The number of valence electrons available for Neon atoms is 8. Neon is situated in Group 18th and has an atomic number of 10.


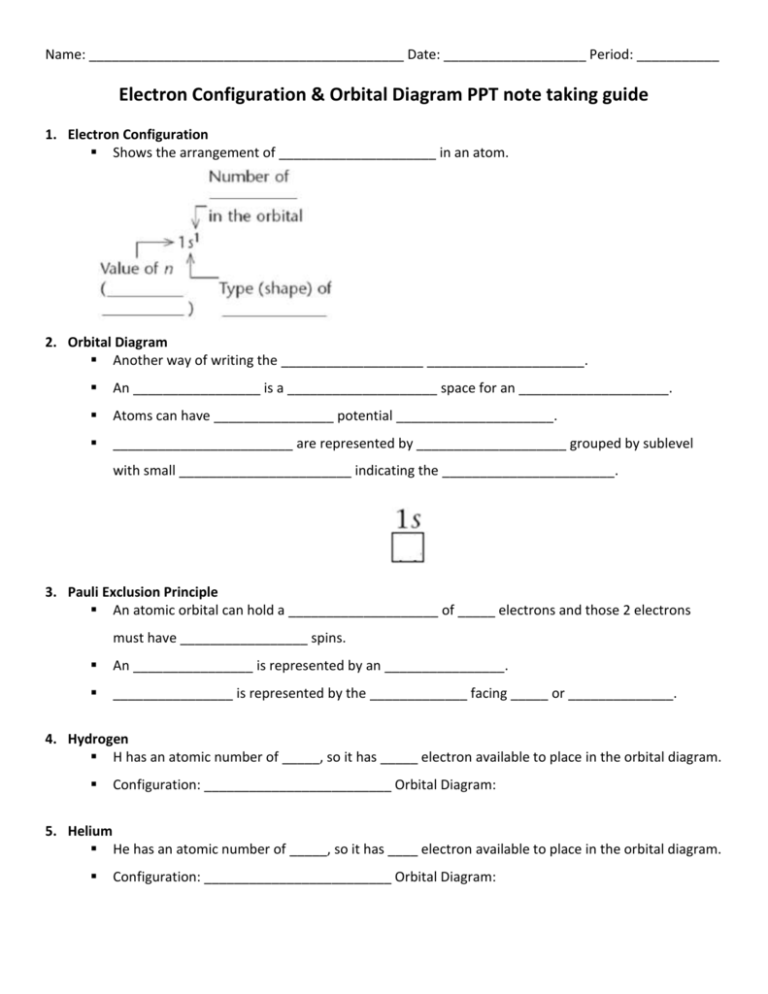
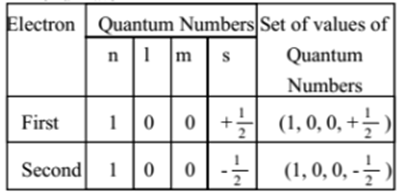

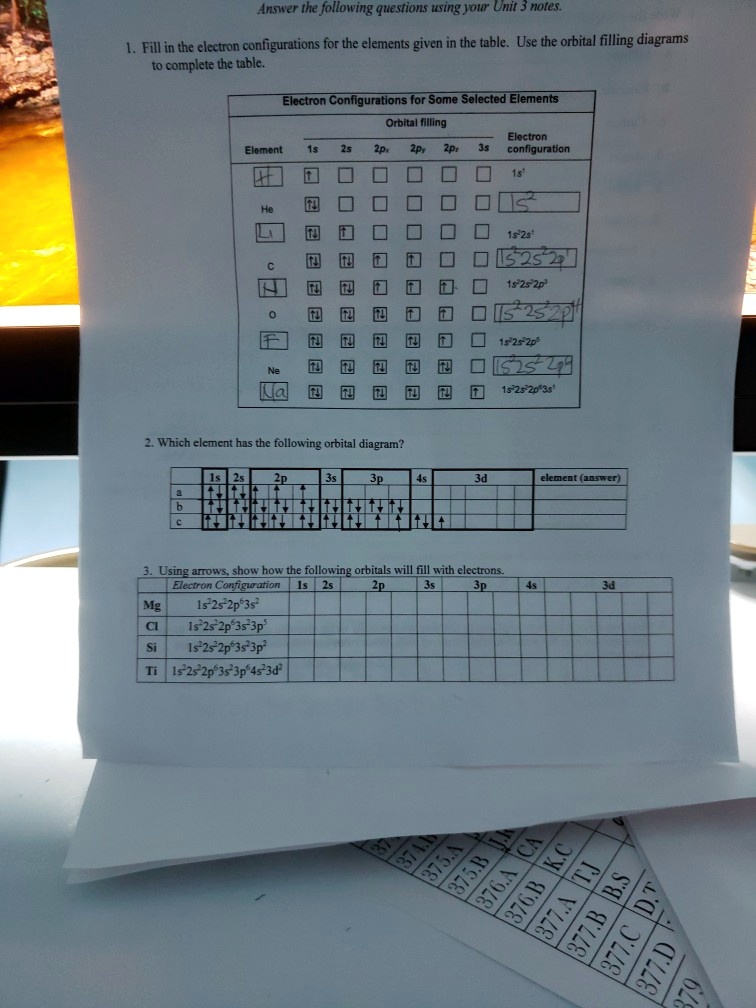




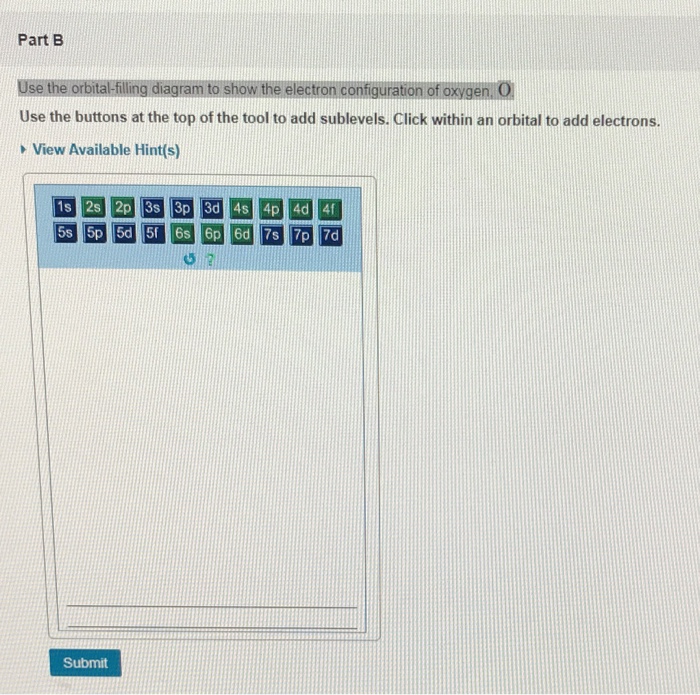











0 Response to "40 Use The Orbital-filling Diagram To Show The Electron Configuration Of Helium, He."
Post a Comment