36 bohr diagram for lithium
Bohr Rutherford Diagram For Sodium What do the Bohr model diagrams for Hydrogen Lithium Sodium and Potassium has in common? they all have one electron in their valence shell. Answered. Below is an illustration of the Bohr model of a sodium atom. If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has. PPT Bohr Diagrams and Lewis Dot Structures Bohr Diagrams and Lewis Dot Structures What you've already learned in class and from readings You learned that Electrons can exist in different energy levels You learned that the # of Electrons in an atom are equal to the # of Protons in an atom You learned that the # of Valence Electrons are the outermost Electrons of an Atom What You're about to learn How to draw the Electrons around an ...
How to draw Lithium Bohr Model - YouTube This video explains on the atomic model structure of Lithium. This is a "Bohr model" on Lithium. #lithium #bohr_model #howtodraw #atomic_model
Bohr diagram for lithium
What is the Bohr model diagram for Lithium? - Answers The Bohr Model: is a diagram of the atom that describes the arrangement of subatomic particles: neutrons and protons in the nucleus and electrons in electron shells. How is Niels Bohr's model of ... Bohr Diagram For Lithium - schematron.org In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr ... Bohr Rutherford Diagram For The First 20 Elements Bohr Rutherford Diagram For The First 20 Elements. The table below shows the electron arrangement for the first 20 elements in the Periodic Table along with their Element, Electrons diagram and configuration. Bohr model of the first 20 elements. Due to the technical limitations of my software, I will list the number of protons and neutrons of ...
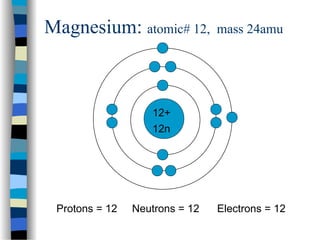
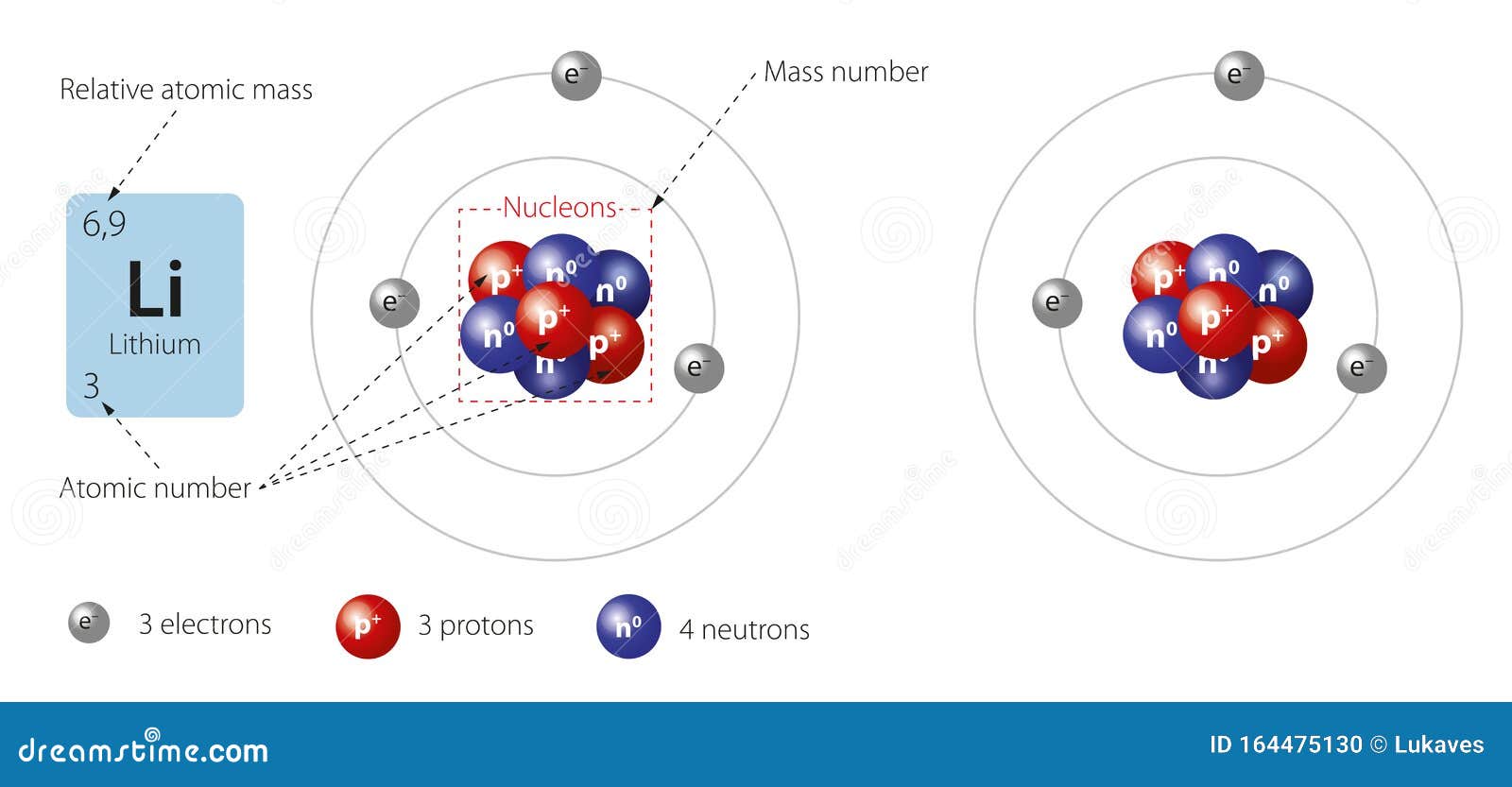
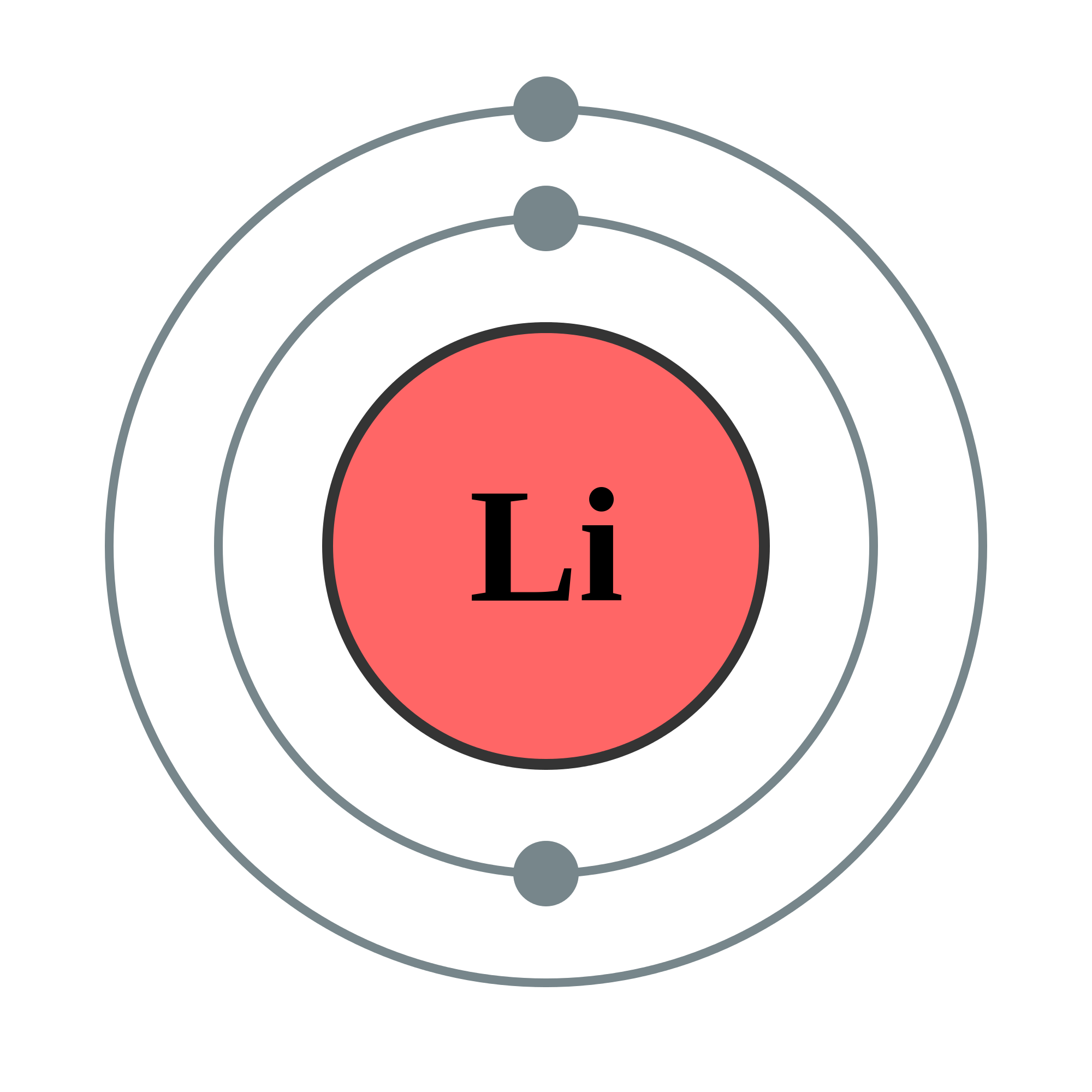
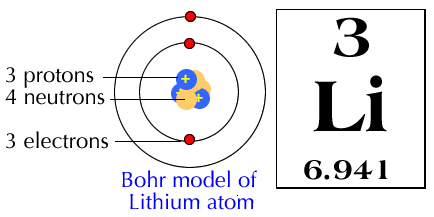
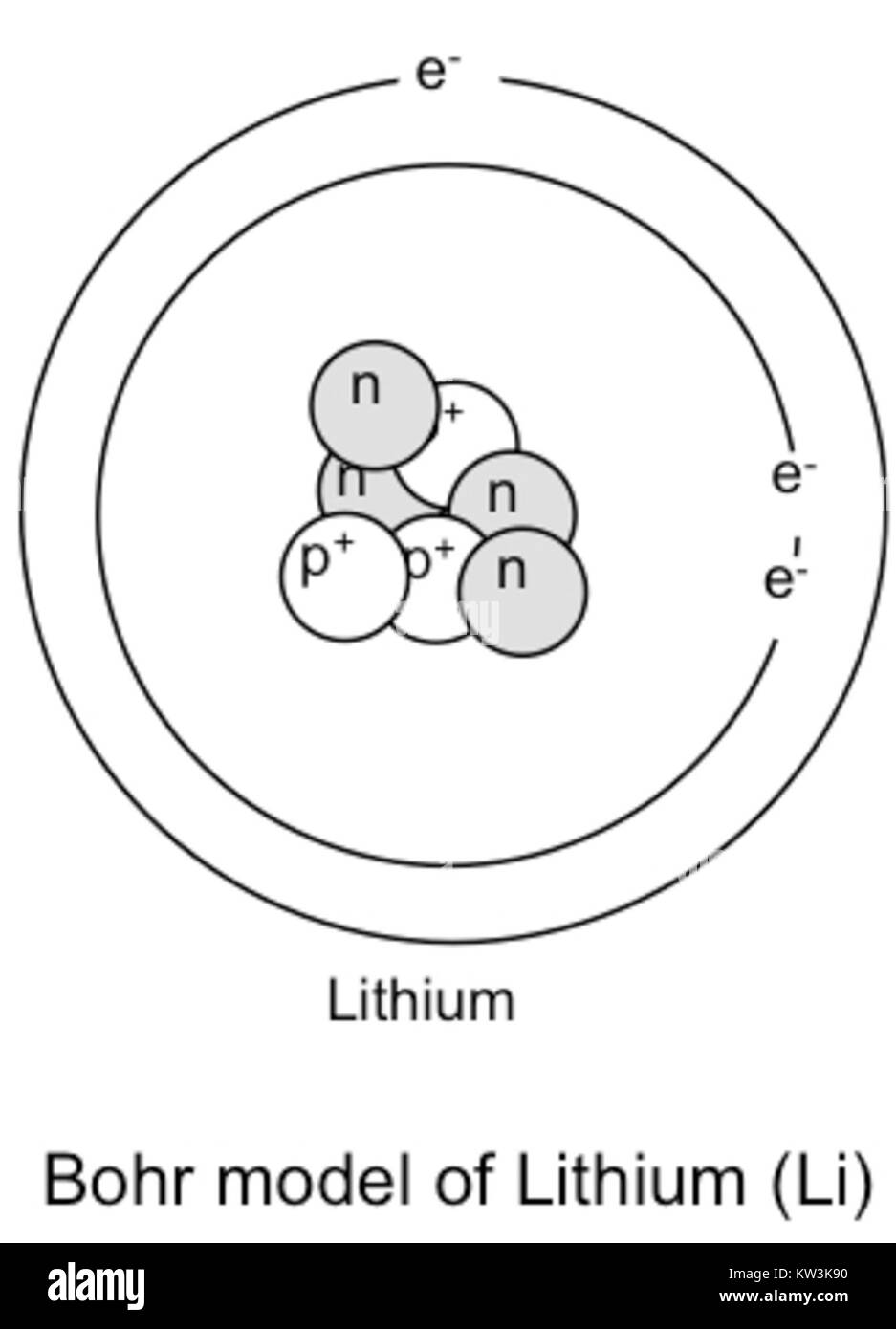
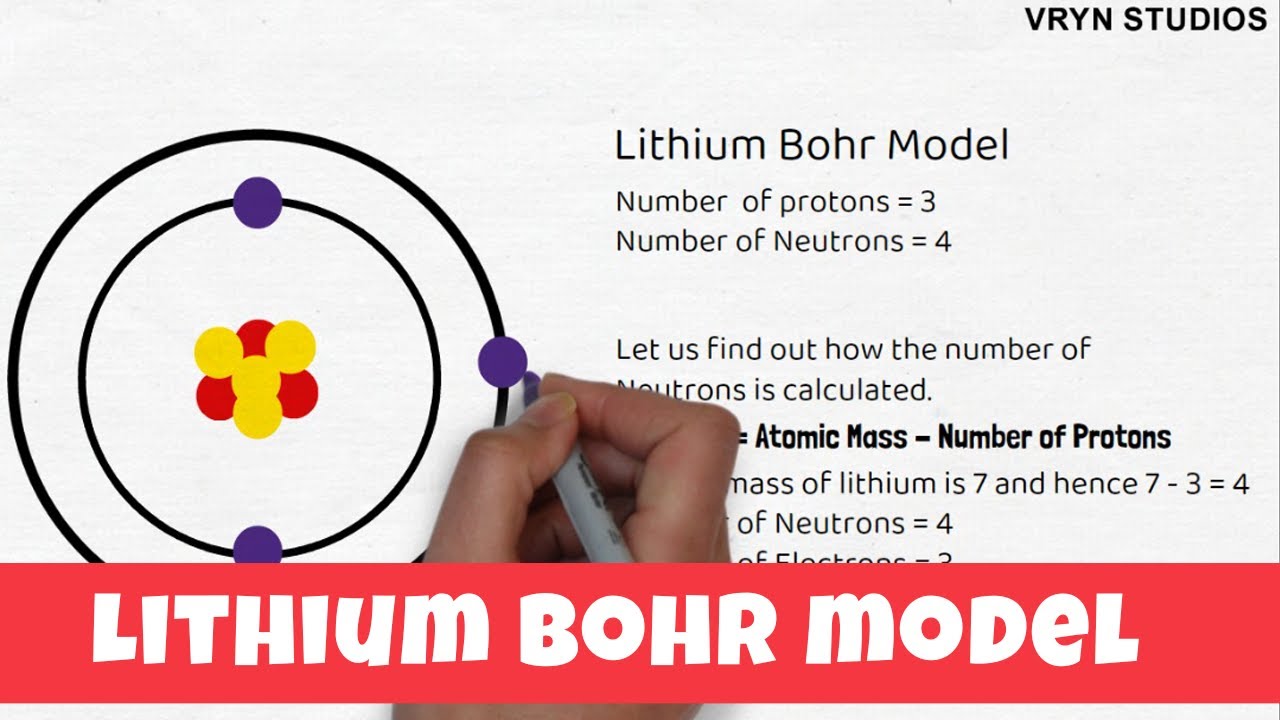
Bohr diagram for lithium. Chromium(Cr) electron configuration and orbital diagram The electron configuration of chromium and the orbital diagram is the main topic in this article. Also, ... configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of chromium(Cr) through … Lithium Bohr Model — Diagram, Steps To Draw - Techiescientist Hence, the final Bohr model of the Lithium atom consists of 3 protons and 4 neutrons inside the nucleus, and 3 electrons revolving around the nucleus. There are 2 electrons present in the K shell and 1 electron in the L shell. Deriving Lewis Diagram from Bohr Model. The Lewis diagram of an atom represents its atomic structure in the pictorial form. PDF Lewis Dot Diagram Worksheet Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Valance Electrons Number of energy levels Bohr Model Lewis Dot Carbon 6 12 6 6 6 4 (group 14, 4 in Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Hydrogen (H) 1: 2: Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11 ...
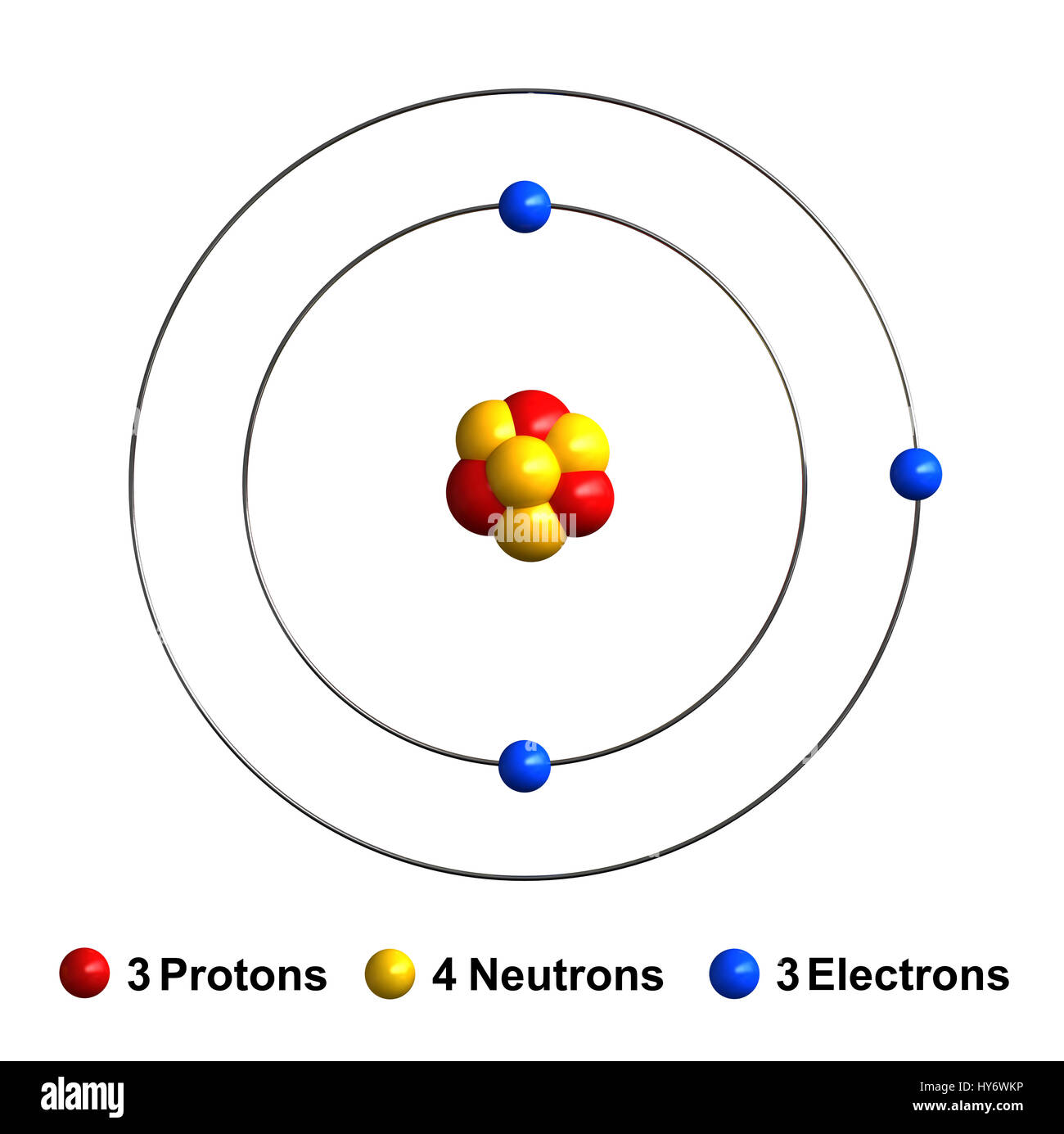
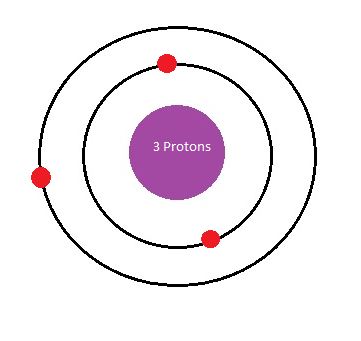
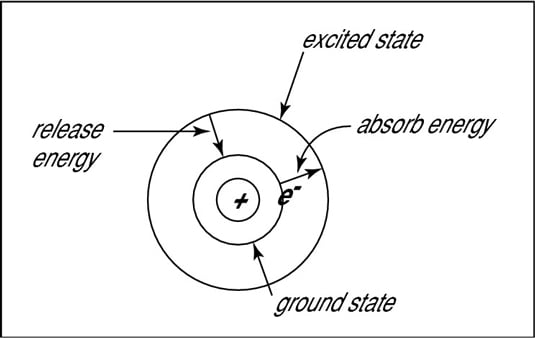
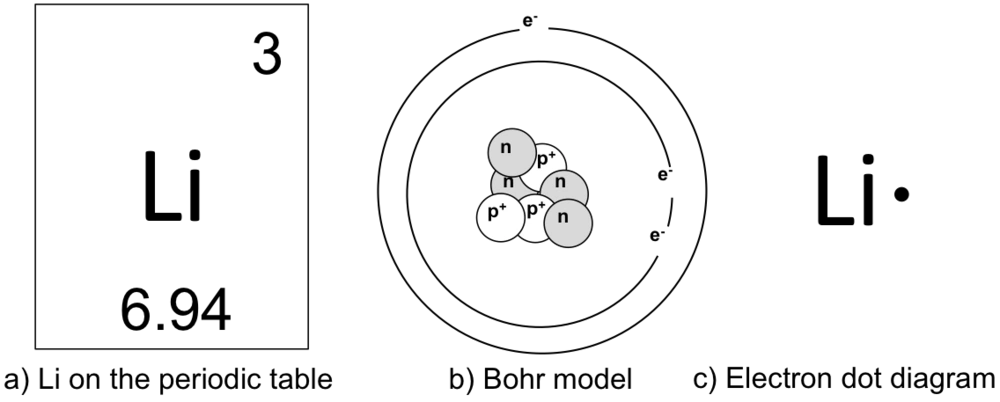
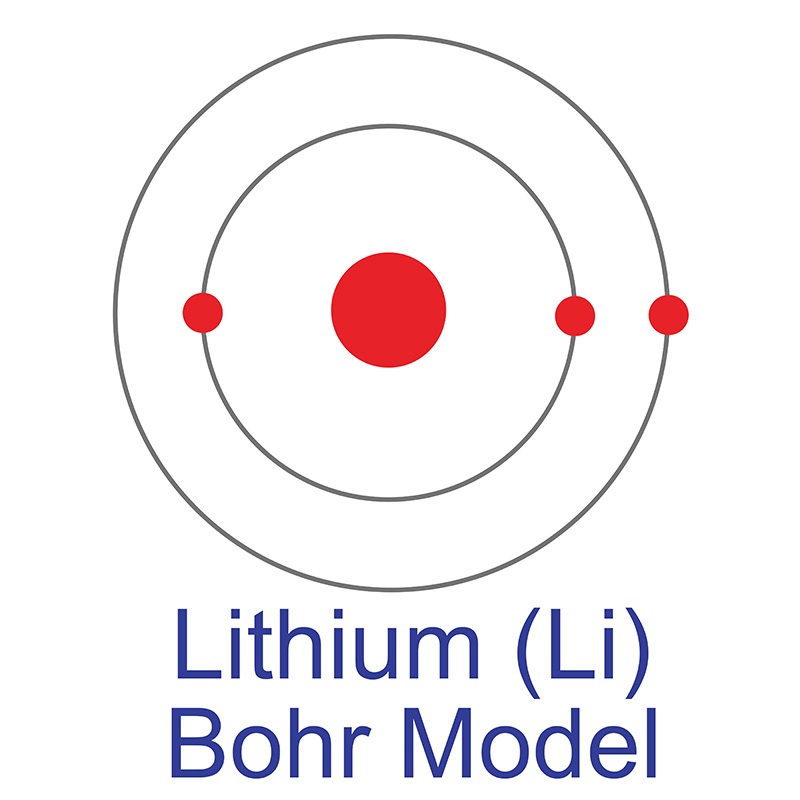
Energy Level - Bohr’s Atomic Model and Postulates of Bohr ... Bohr's Model explained how electrons travel in different circular orbits around the nucleus. The orbits are symbolized with the letter ‘n’, where the value of n is an integer. The transfer of electrons is possible by emission and absorption of energy. Viewing the demerits of the Rutherford model, Neil Bohr concluded that classical mechanics and electromagnetism cannot … Lithium Bohr Model - How to draw Bohr diagram for Lithium ... The Bohr Model of Lithium(Li) has a nucleus that contains 4 neutrons and 3 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Lithium contains only 1 electron that also called valence electron. What is the Bohr diagram for lithium? Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Lithium has three electrons: two go to K shell and. lithium bohr diagram - theseventhart.org lithium bohr diagram. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. And in the orbit of Fig. the wavelength of the photon given off is given by. So approximately, we can suppose the 2S electron is moving around the e+ nucleus ( +3e - 2e = +e ) on the circular orbit of the two de Broglie ...
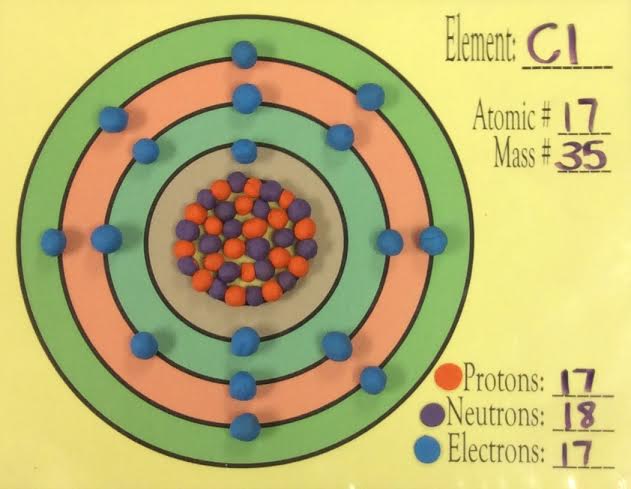
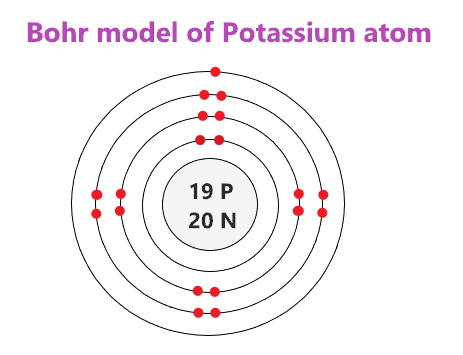
bohr diagram Flashcards and Study Sets - Quizlet What is the pattern for the first 4 she…. Shows how many electrons each shell has. Niels Bohr. The number of protons and neutrons are shown in the centre and…. 2, 8, 8, 18. Bohr Diagram. Shows how many electrons each shell has. Named after. Niels Bohr. Chlorine Bohr Model - How to draw Bohr diagram for ... Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Chlorine atom with some simple steps. Steps to draw the Bohr Model of Chlorine atom . 1. Find the number of protons, electrons, and neutrons in the Chlorine atom. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the … how to make a bohr diagram - shapovmusic.com Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. …. Set up the diagram. To set up the diagram, you will need a circle in the middle. …. Add in orbitals and electrons. Potassium Bohr Model - How to draw Bohr diagram for ... The Bohr Model of Potassium(K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron.
How many electrons are in the Bohr diagram of lithium ... What is the Bohr diagram of lithium? The Bohr model of Lithium is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains only 1 electron. Lithium is neutral and its atomic number is 3, hence, the number of protons and electrons available for its Bohr diagram is also 3.
Bohr Rutherford Diagram For Hydrogen - schematron.org There are two ways in which one can observe spectral lines from an Rutherford analyzed the scattering data and developed a . draw a Bohr-Rutherford diagram for hydrogen. draw a Bohr-Rutherford diagram for helium. draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for a Bohr-Rutherford diagram is used to show the numbers ...
PDF Bohr Model Worksheet - Duplin County Schools Bohr Model Worksheet For each element draw the inner electrons blue & the valence (outer) ... The Structure of Atoms Complete the table Sub-atomic Particle Symbol Location in the atom Mass of particle ... Lithium Li 3 7-3=4 3 carbon C 6 12-6=6 6 Sodium Aluminum Pb Ti Zn 80 17 Tungsten . Title: Bohr Model Worksheet ...
Bohr model - Wikipedia The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else.
Bohr's model - SlideShare How to-bohr-diagram Nikki Wilkinson. How to draw bohr diagrams (slideshare) dumouchelle. Recommended. Bohr Theory Gargi Education ... 1e +1 Beryllium Ion 4p, 1e +3 Lithium Ion 3p, 1e +2 40. 4- If a substance which gives line emission spectrum is placed in a magnetic field, the lines of the spectrum get split up into a number of closely spaced lines. This phenomenon is known …
Aufbau Principle with Exceptions - ChemistryGod 2020-02-18 · Aufbau principle. Each type of orbitals in the above diagram is colored the same and are arranged in the ascending order of the principal quantum number (n) from the top to bottom, for example, 2p, 3p, 4p, 5p, 6p…From the left to right, the orbitals are arranged according to the azimuthal quantum number (l).l = 0 corresponds to the s orbital, l = 1 corresponds to the …
BOHR'S DIAGRAM | Chemistry Quiz - Quizizz answer choices. The bohr model diagram represents all the subatomic particles, while the lewis dot diagram only shows the symbol and the valence electrons. The lewis dot shows all the electrons and the bohr model only shows the electrons in the last shell. The lewis dot shows the number of neutrons and the bohr model only shows the electrons.
Bohr model of Lithium - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
How to Draw Lithium Bohr Model - Your Home Teacher It shows the number of Protons, Neutrons and Electrons. You can also learn how to calculate Neutrons from Protons and Atomic Mass.Oct 8, 2020 · Uploaded by VRYN Studios
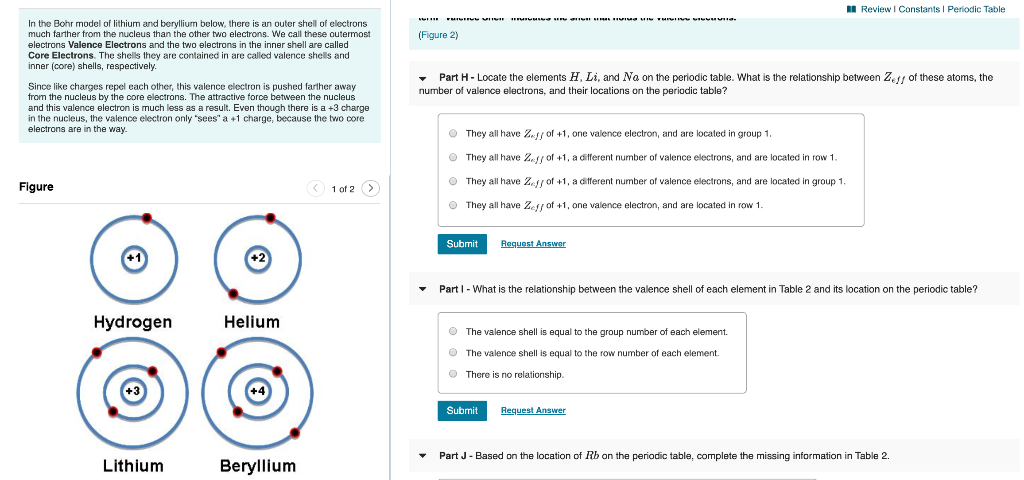
(a) Draw the Bohr-Rutherford diagram (without neutrons ... Find step-by-step Biology solutions and your answer to the following textbook question: (a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.
Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.
Bohr's Lithium(ion) Li(+) - BIGLOBE Naturally occurring lithium is composed of two stable isotopes, Li6 and Li7, the latter being the more abundant (92.5%). Bohr model Lithium ion (Li+) The ionization energies of the lithium is 5.39 eV (1st), 75.64 eV (2nd), and 122.45 eV (3rd), respectively. So the ground state energy of the lithium ion (Li+) is -75.64 - 122.45 = -198.09 eV.
Lewis Dot Structures Worksheet - Mr. Walsh's Class Bohr Diagram. Lewis Dot Structure. Ne. electron config # protons. #electrons. charge. Bohr Diagram. Lewis Dot . Making Ions – Ionic Bonds are made of Ions. A strong understanding of Ions is needed. Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive “cations”. Nonmetals tend to gain electrons, …
How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He c) O d) Al e) Ne f) K 6p 6n. Bohr Diagrams …
Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
Bohr Rutherford Diagram For The First 20 Elements Bohr Rutherford Diagram For The First 20 Elements. The table below shows the electron arrangement for the first 20 elements in the Periodic Table along with their Element, Electrons diagram and configuration. Bohr model of the first 20 elements. Due to the technical limitations of my software, I will list the number of protons and neutrons of ...
Bohr Diagram For Lithium - schematron.org In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr ...
What is the Bohr model diagram for Lithium? - Answers The Bohr Model: is a diagram of the atom that describes the arrangement of subatomic particles: neutrons and protons in the nucleus and electrons in electron shells. How is Niels Bohr's model of ...
















0 Response to "36 bohr diagram for lithium"
Post a Comment