36 lewis diagram for co
CO2 (Carbon dioxide) Lewis Structure and Shape CO 2 lewis structure and Shape In the lewis structure of CO 2, you can see there are two double bonds around carbon atom. Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Also, there are no charges in oxygen atoms and carbon atom. CO Lewis Structure - Lewis Dot Structure | Chem Helps To draw Lewis Structure of CO, we need to know the valence electrons of carbon and valence electrons of oxygen. Valence electrons of C is 4 while valence electrons of O is 6. Carbon and oxygen needs to form a triple bond between them to satisfy the octet rule. It means that 6 of the valence electrons are used in the triple bond.
What is the Lewis structure of CO? | Socratic This often looks wrong to a student who is used to seeing double bonds on oxygen. Students are typically taught an electron-counting method, which goes as follows: Count the number of valence electrons per atom. Draw out a predicted atom connectivity. Place all electrons in predicted spots. Where there are electron pairs, construct one bond line for each electron pair.

Lewis diagram for co
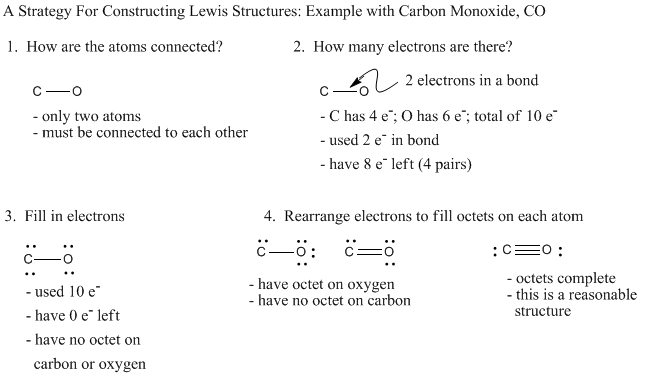
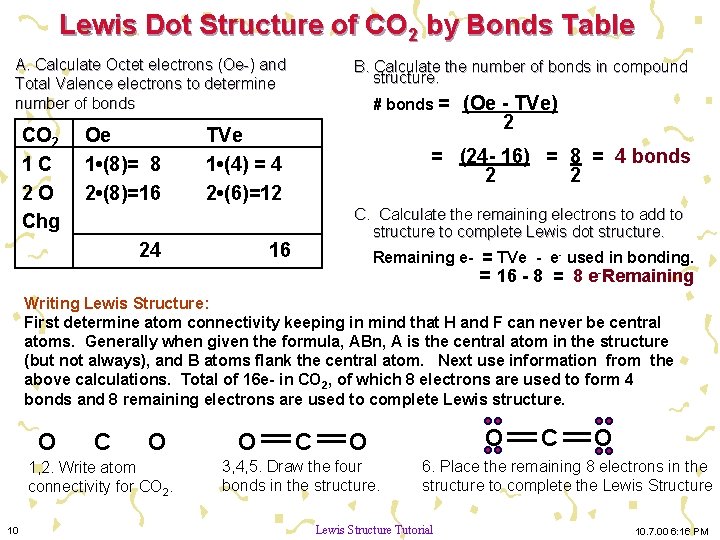
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. CO Lewis structure, Hybridization, and Molecular Geometry ... CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen. topblogtenz.com › carbon-dioxide-co2-lewisCO2 lewis structure, molecular geometry, bond angle, polar or ... In the CO2 lewis structure, there are a total of 4 lone pairs means 8 nonbonding electrons are present. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Let’s see how to draw a CO2 lewis dot structure with simple steps. Follow some steps for drawing the Lewis dot structure for CO2 1.
Lewis diagram for co. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure of CO (Carbon Monoxide) A carbon monoxide molecule consists of one carbon atom and one oxygen atom. The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two. Therefore, the valency is satisfied via the donation of a lone pair of electrons for bonding by the oxygen atom. How to Draw the Lewis Dot Diagram for Carbon monoxide (CO ... te them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of CO structure there are a total of 10 valence... Lewis Structures: Dot Symbols, Diagrams, Examples, Questions Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons. O = 6 × 2 = 12 valence electrons. Draw the Lewis structure for CO - Ask4Essay A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom.
What is the Lewis Structure of CO? - Quora 3 Dec 2017 — Carbon monoxide is considered a Lewis base because it is well known to react with transition metals to form metal carbonyls. In those reactions , It donates a ...3 answers · 14 votes: Usually shown as There are various ways of configuring the electrons. Formal charge Suggests ...How to determine the Lewis dot structure of carbon ...1 answer21 Oct 2017What is the Lewis dot diagram for carbon monoxide ...2 answers19 Aug 2016In the Lewis structure of CO, how is it possible that ...3 answers25 Jun 2017What is the Lewis dot diagram for carbon dioxide ...1 answer31 May 2016More results from CO2 Lewis Structure (2021 UPDATED) All You Need To Know CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula. CO Lewis Structure & Molecular Geometry - What's Insight In a CO lewis structure, the overall ratio of carbon to the oxygen atom is 1:1. Carbon forms three tipple bonds with oxygen by sharing three valence ...Molecular Geometry of CO: LinearHybridization of CO: sp hybridizationC-O bond distance: 112.8 pmThe dipole moment of CO: 0.122 D › definition-of-lewis-structureLewis Structure Definition and Example - ThoughtCo Oct 16, 2019 · A Lewis structure is a diagram that shows the covalent bonds and lone electron pairs in a molecule. Lewis structures are based on the octet rule. While Lewis structures are useful for describing chemical bonding, they are limited in that they do not account for aromaticity, nor do they accurately describe magnetic behavior.
CO Lewis Structure, Geometry, and Hybridization ... 15-03-2022 · CO Lewis Structure, Geometry, and Hybridization Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. American Financing Corporation - Better Business Bureau BBB accredited since 1/30/2002. Mortgage Banker in Aurora, CO. See BBB rating, reviews, complaints, request a quote & more. MO diagram and Lewis Structure of CO - CHEMISTRY COMMUNITY Re: MO diagram and Lewis Structure of CO Post by 104277942 » Fri Dec 06, 2013 7:36 am For bond order it is a good rule of thumb that triple bonds = 3 double = 2 and single = 1 but if you want to be sure, I personally like drawing a quick MO diagram sketch on the side and do the formula (bonding-antibonding)/2 to find the bond order if I am not ... Lewis diagrams (practice) | Khan Academy Lewis diagrams. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Exceptions to the octet rule. Worked example: Lewis diagram of xenon difluoride (XeF₂) Practice: Lewis diagrams. This is the currently selected item. Next lesson.
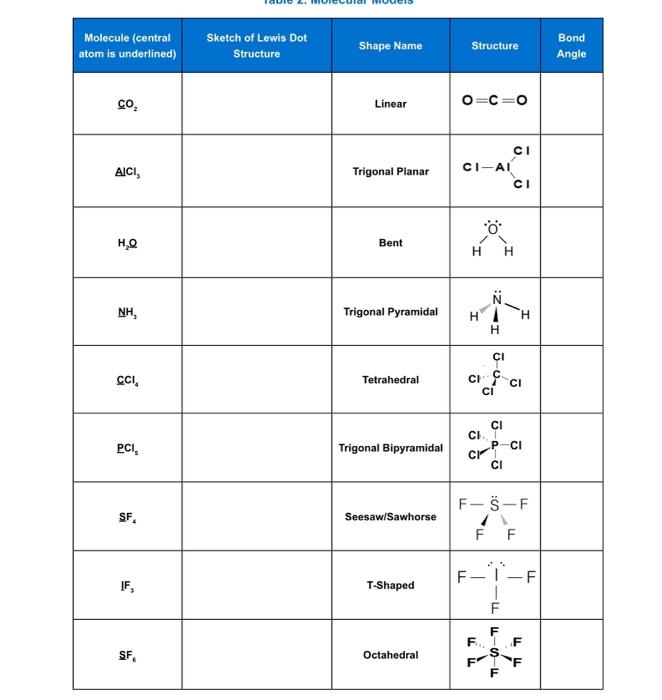
chem.libretexts.org › Bookshelves › Physical_andGeometry of Molecules - Chemistry LibreTexts Aug 21, 2020 · Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the ...
Draw the lewis structure of co - fornoob.com Complete the octet of carbon to get the Lewis structure of CO. C has four electrons in step 4 and thus, has an incomplete octet. Therefore, change two lone pairs of O to bonding pairs to form two double bonds. This completes the octet of C and hence, the octet of each atom is completed and Lewis structure of CO is obtained. Step 6 of 6
What is the Lewis Structure of CO? - Quora Answer (1 of 3): Usually shown as There are various ways of configuring the electrons. Formal charge Suggests that things may be more complicated.
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure. Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen.
CO2 Lewis Structure - Easy Hard Science The first thing about the CO 2 Lewis structure is to put carbon in the center. Make both O atoms connect to C. There won't be any bonds between the O's directly. As a rule, carbon is always going to be in the center, and the other atoms connect to it. Second, connect the lone dots on each O to the C in the center. Each O needs to bond twice.
Write the Lewis dot structure of CO molecule class 11 ... Let us now write the electron dot structure also called as Lewis dot structure for C O molecule. - Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
Lewis Structure for CO - TerpConnect Lewis Structure for CO Lewis Structure for CO Commonly Tested Lewis Structures We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for CO
Lewis - Wikipedia Names. Lewis (given name), including a list of people with the given name Lewis (surname), including a list of people with the surname Music. Lewis (musician), Canadian singer "Lewis (Mistreated)", a song by Radiohead from My Iron Lung Places. Lewis (crater), a crater on the far side of the Moon Isle of Lewis, the northern part of Lewis and Harris, Western Isles, Scotland
Lewis Structure of CO (Carbon Monoxide) - YouTube The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill oute...
Solved 7. Draw the Lewis structures and molecular orbital ... 7. Draw the Lewis structures and molecular orbital diagrams for Co and NO. What are their bond orders? Are the molecular orbital diagram similar to their Lewis structures? Explain. co Lewis Structure NO Lewis Structure CO Bond Order NO Bond Order co Molecular Orbital Diagram NO Molecular Orbital Diagram ö ; Question: 7. Draw the Lewis ...
How to Draw a Lewis Structure - ThoughtCo A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
Carbon monoxide (CO) Molecule Lewis Structure Carbon monoxide (CO) is a diatomic molecule and contains carbon and oxygen atoms. Lewis structure of CO molecule contains a triple bond. Both Carbon and Oxygen atoms have one lone pair in their valence shells. CO lewis structure In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells.
Lewis Structure Practice Worksheet And Answers - Practice ... Lewis Structure Practice Worksheet And Answers - Pre-algebra worksheets can be located online on many sources. These worksheets consist of word problems, formulas, as well as Usual Core standards. For evaluation, print these resources and confirm your answers.
Lewis structure calculator | Lewis structure generator These type diagrams are a simplified representation of the valence shell electrons in a molecule. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds. The reason is that electrons are shared in a covalent bond. In an ionic bond, it is more like one atom donating an electron to the other atom.
study.com › academy › lessonLewis Structures: Single, Double & Triple Bonds - Video ... Nov 21, 2021 · Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows the valence electrons in an element.
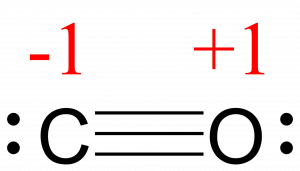
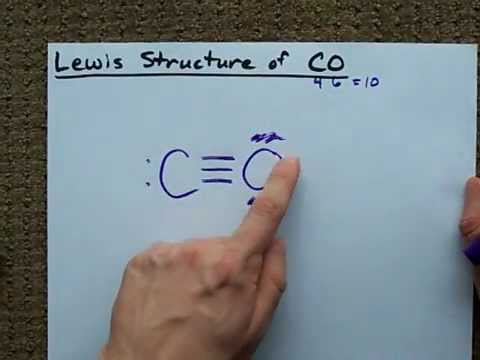
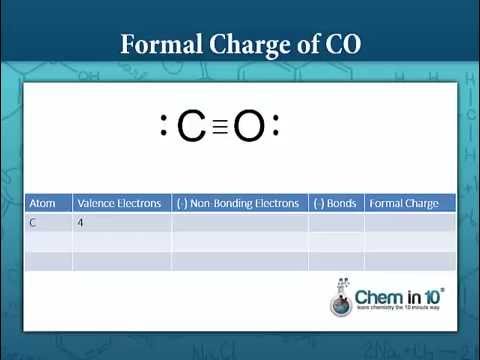
Write the Lewis dot structure of CO molecule. The lewis dot structure of carbon monoxide is: :C≡O: Solve any question of Chemical Bonding and Molecular Structure with:-.
techiescientist.com › co32-lewis-structureCO32- Lewis Structure, Molecular Geometry, Hybridization, and ... Mar 18, 2022 · Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence Electrons.
CO Lewis Structure: How to Draw the Dot Structure for CO ... In the CO Lewis structure there aren't enough valence electrons available for each atom to obtain an octet without sharing more than one pair. Therefore CO has a triple bond between the carbon and oxygen atom. For the CO Lewis structure there are a total of 10 valence electrons available.
PDF LEWIS DIAGRAMS - Colorado State University Lewis diagrams, you would fi nd that the triangular form for ozone works well but it is excluded by experimental evidence. Therefore we must procede to work out the linear alternative: There are a total of 6 x 3 = 18 valence electrons, of which 4 are used in the single bonds, leaving 14. By
In the Lewis structure of CO, how is it possible that ... Answer (1 of 3): This more or less boils down to fulfilling the octet rule for CNOF… elements. So oxygen shares 4 electrons within the triple bond and carbon shares 2, however, the bond is considered polar covalent (the electrons spend more time orbiting oxygen). This is due to the higher electro...
Herbert Spencer’s Theory of Social Evolution (Explained ... ADVERTISEMENTS: The most important contribution of Herbert Spencer to Sociology is the theory of evolution. He utilized the principles of physical and biological evolution in order to elaborate and explain his theory of Social evolution. In physical evolution, a movement is from indefinite incoherent situation to definite and coherent situation. Besides, the underlying …
topblogtenz.com › carbon-dioxide-co2-lewisCO2 lewis structure, molecular geometry, bond angle, polar or ... In the CO2 lewis structure, there are a total of 4 lone pairs means 8 nonbonding electrons are present. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Let’s see how to draw a CO2 lewis dot structure with simple steps. Follow some steps for drawing the Lewis dot structure for CO2 1.
CO Lewis structure, Hybridization, and Molecular Geometry ... CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
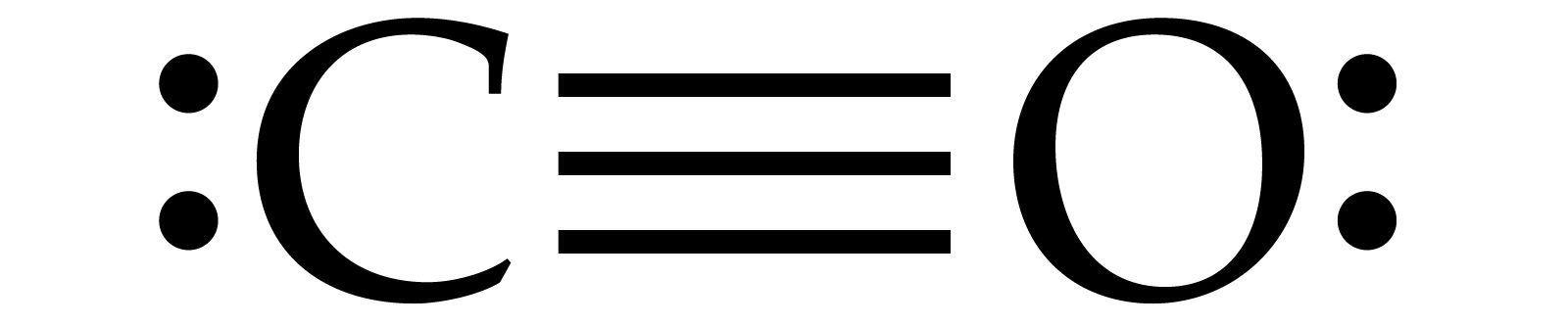








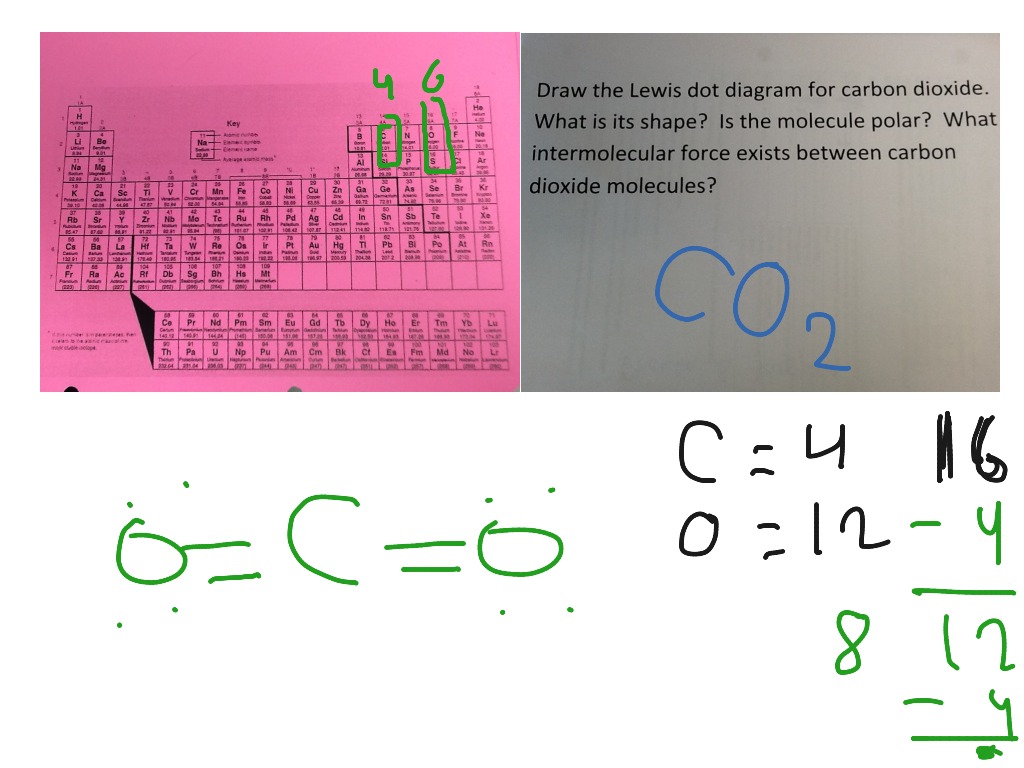
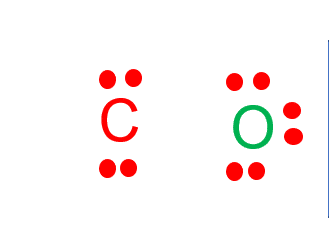
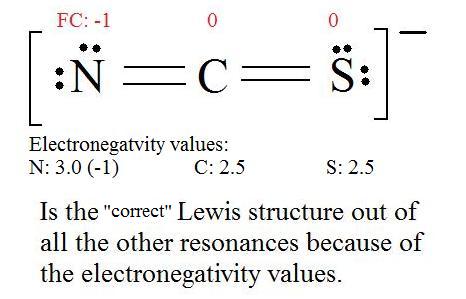










![Sample Paper Term 2] The table shows electronic structures of ...](https://d1avenlh0i1xmr.cloudfront.net/00ce2470-0895-455a-8597-a5192013f394/lewis-structure-of-co-01.jpg)
0 Response to "36 lewis diagram for co"
Post a Comment