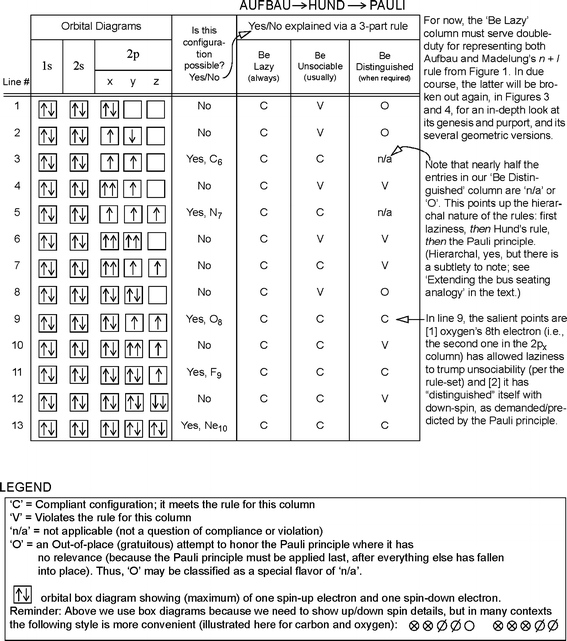
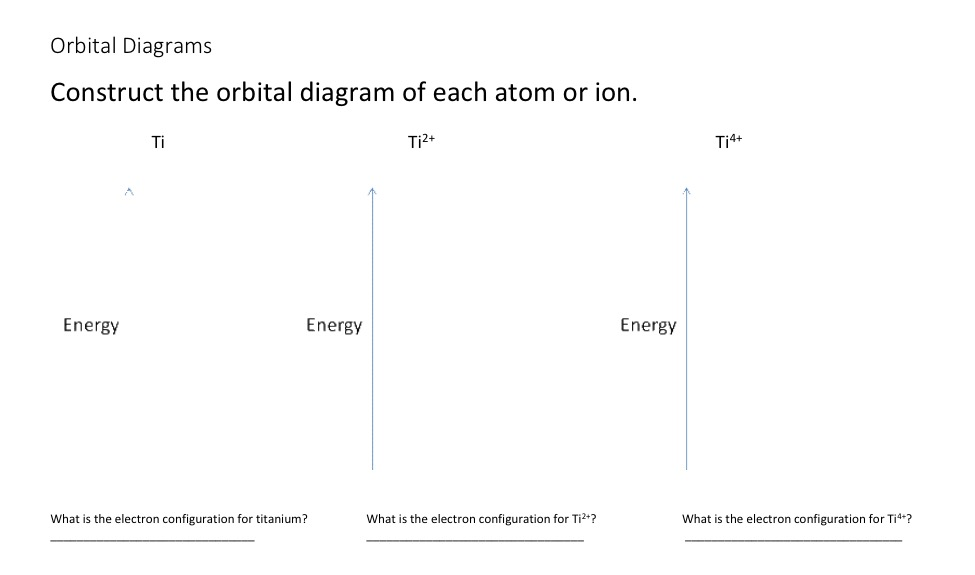
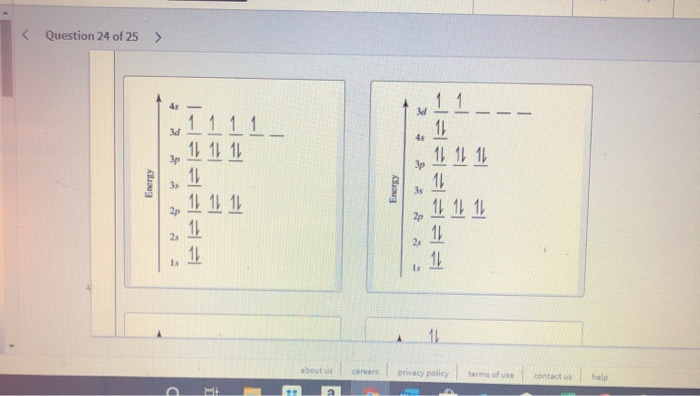
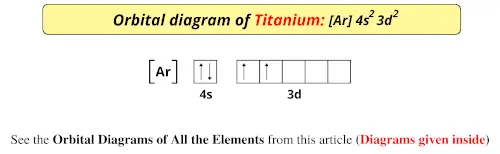
36 orbital diagram for ti
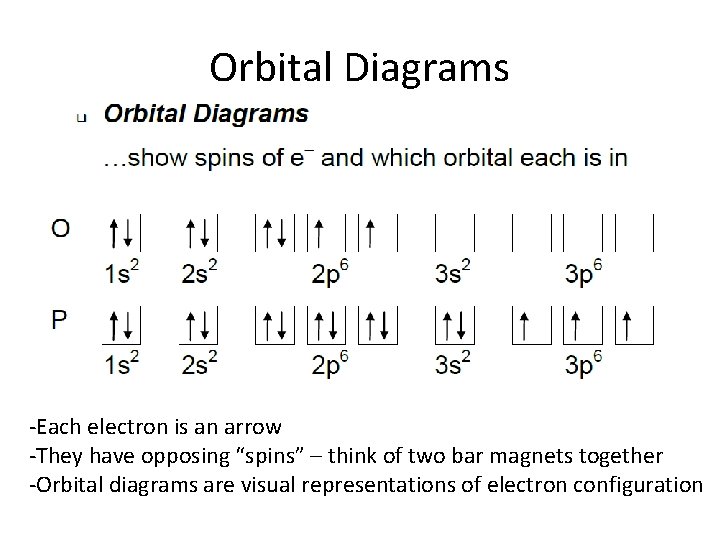
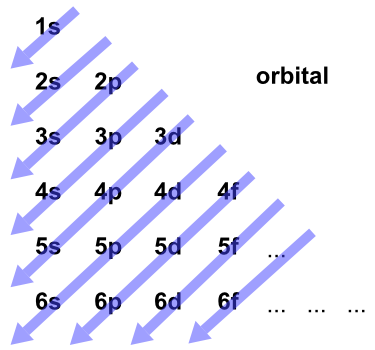
NCERT Solutions for Class 12 Physics in PDF updated for ... Draw a labelled diagram of AC generator. Derive the expression for the instantaneous value of the emf induced in the coil. A circular coil of cross-sectional area 200 sq. cm and 20 terns is rotated about the vertical diameter with angular speed of 50 rad/s in a uniform magnetic field of magnitude 3.0 × 10^-2 T. Calculate the maximum value of the current in the coil. [Delhi 2017] 6.4 Electronic Structure of Atoms (Electron Configurations ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3d orbital is higher in energy than …
› learn › latexTikZ package - Overleaf, Online LaTeX Editor TikZ is probably the most complex and powerful tool to create graphic elements in L a T e X. Starting with a simple example, this article introduces some basic concepts: drawing lines, dots, curves, circles, rectangles etc.

Orbital diagram for ti
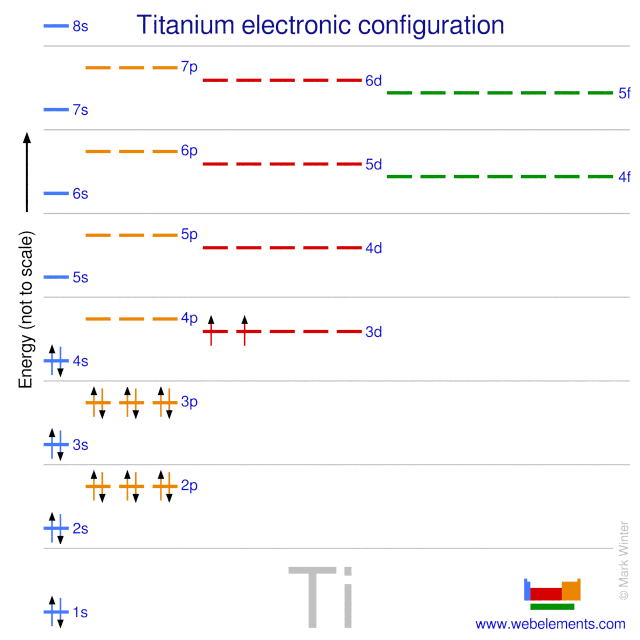
Product selectivity of photocatalytic CO2 ... - ScienceDirect 01.01.2020 · Photocatalytic carbon dioxide (CO 2) reduction to obtain hydrocarbon solar fuels is one of the promising strategies to solve energy crisis and complement carbon cycle.However, the low activity and poor product selectivity greatly limit its practical application. Tuning product selectivity is of great significance to improve the yield of target product and deepen the … Hückel's Rule - Chemistry LibreTexts 12.09.2020 · You can see how this works with the molecular orbital diagram for the aromatic compound, benzene, below. Figure 1: Molecular Orbitals levels of Benzene. Benzene has 6 \(\pi\) electrons. Its first 2 \(\pi\) electrons fill the lowest energy orbital, and it has 4 \(\pi\) electrons remaining. These 4 fill in the orbitals of the succeeding energy level. Notice how all of its … chem.libretexts.org › Bookshelves › General_Chemistry5.17: Electron Configurations and the Periodic Table ... Jul 19, 2021 · The transition elements or transition metals are those elements whose distinguishing electron is found in a d orbital. The first examples of transition metals (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) are found in the fourth period even though the distinguishing electron in each case is a 3 d electron and belongs to the third shell.
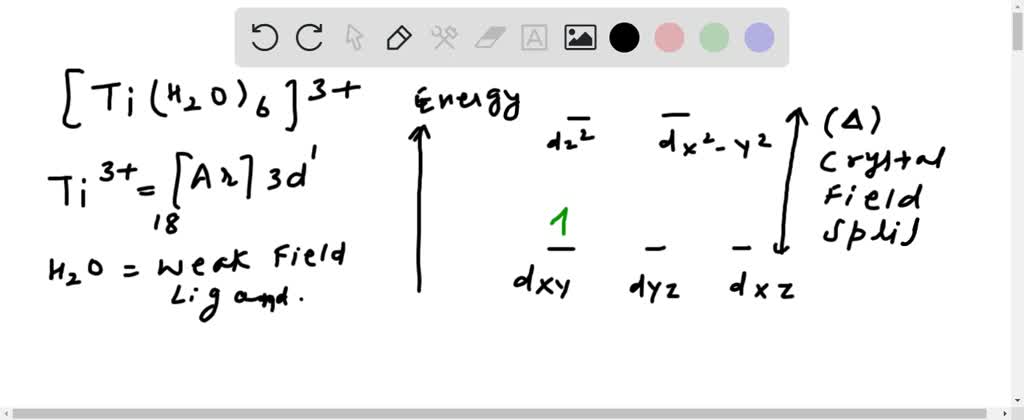
Orbital diagram for ti. › educmat › chm2060_preussGround State Electron Configurations - University of Guelph Angular momentum l (orbital shape) Magnetic m l (orbital orientation) These 3 quantum numbers are the spatial quantum numbers. ⇒ together, they describe the 3D appearance of the orbital in space ⇒ the spatial probability distribution of an e-described by that orbital The 4th quantum number is necessary to fully describe an e-in an orbital. chemed.chem.purdue.edu › genchem › topicreviewQuantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. en.wikipedia.org › wiki › Tanabe–Sugano_diagramTanabe–Sugano diagram - Wikipedia There is no electron repulsion in a d 1 complex, and the single electron resides in the t 2g orbital ground state. A d 1 octahedral metal complex, such as [Ti(H 2 O) 6] 3+, shows a single absorption band in a UV-vis experiment. The term symbol for d 1 is 2 D, which splits into the 2 T 2g and 2 E g states. chem.libretexts.org › Bookshelves › General_Chemistry5.17: Electron Configurations and the Periodic Table ... Jul 19, 2021 · The transition elements or transition metals are those elements whose distinguishing electron is found in a d orbital. The first examples of transition metals (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) are found in the fourth period even though the distinguishing electron in each case is a 3 d electron and belongs to the third shell.
Hückel's Rule - Chemistry LibreTexts 12.09.2020 · You can see how this works with the molecular orbital diagram for the aromatic compound, benzene, below. Figure 1: Molecular Orbitals levels of Benzene. Benzene has 6 \(\pi\) electrons. Its first 2 \(\pi\) electrons fill the lowest energy orbital, and it has 4 \(\pi\) electrons remaining. These 4 fill in the orbitals of the succeeding energy level. Notice how all of its … Product selectivity of photocatalytic CO2 ... - ScienceDirect 01.01.2020 · Photocatalytic carbon dioxide (CO 2) reduction to obtain hydrocarbon solar fuels is one of the promising strategies to solve energy crisis and complement carbon cycle.However, the low activity and poor product selectivity greatly limit its practical application. Tuning product selectivity is of great significance to improve the yield of target product and deepen the …























0 Response to "36 orbital diagram for ti"
Post a Comment