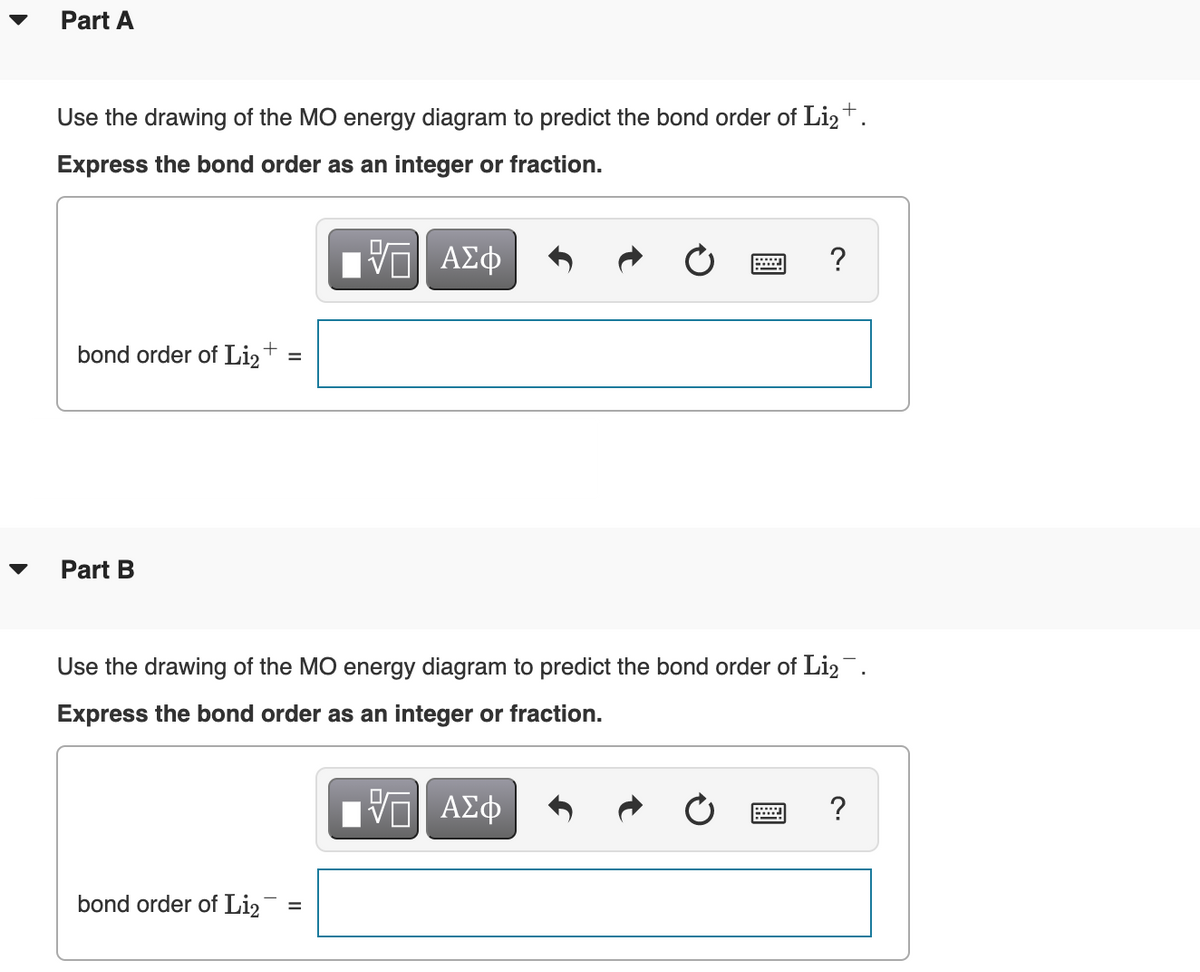
36 use the drawing of the mo energy diagram to predict the bond order of li2+
Use the drawing of MO energy diagram to predict the bond ... Answer to: Use the drawing of MO energy diagram to predict the bond order of L i + 2 and L i 2 . 1. Do you expect L i + 2 to exist in the...1 answer · Top answer: • Bond order of Li+2Li2+ Since Li atom (1s22s2)(1s22s2)has three... PDF MO Diagrams for Diatomic Molecules - UCI Department of ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Answered: Use the drawing of MO energy diagram… | bartleby Solution for Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) close. Start your trial now! First week only $4.99! ... Use the MO diagrams to calculate the bond order for Li2+ and Li2− ...

Use the drawing of the mo energy diagram to predict the bond order of li2+
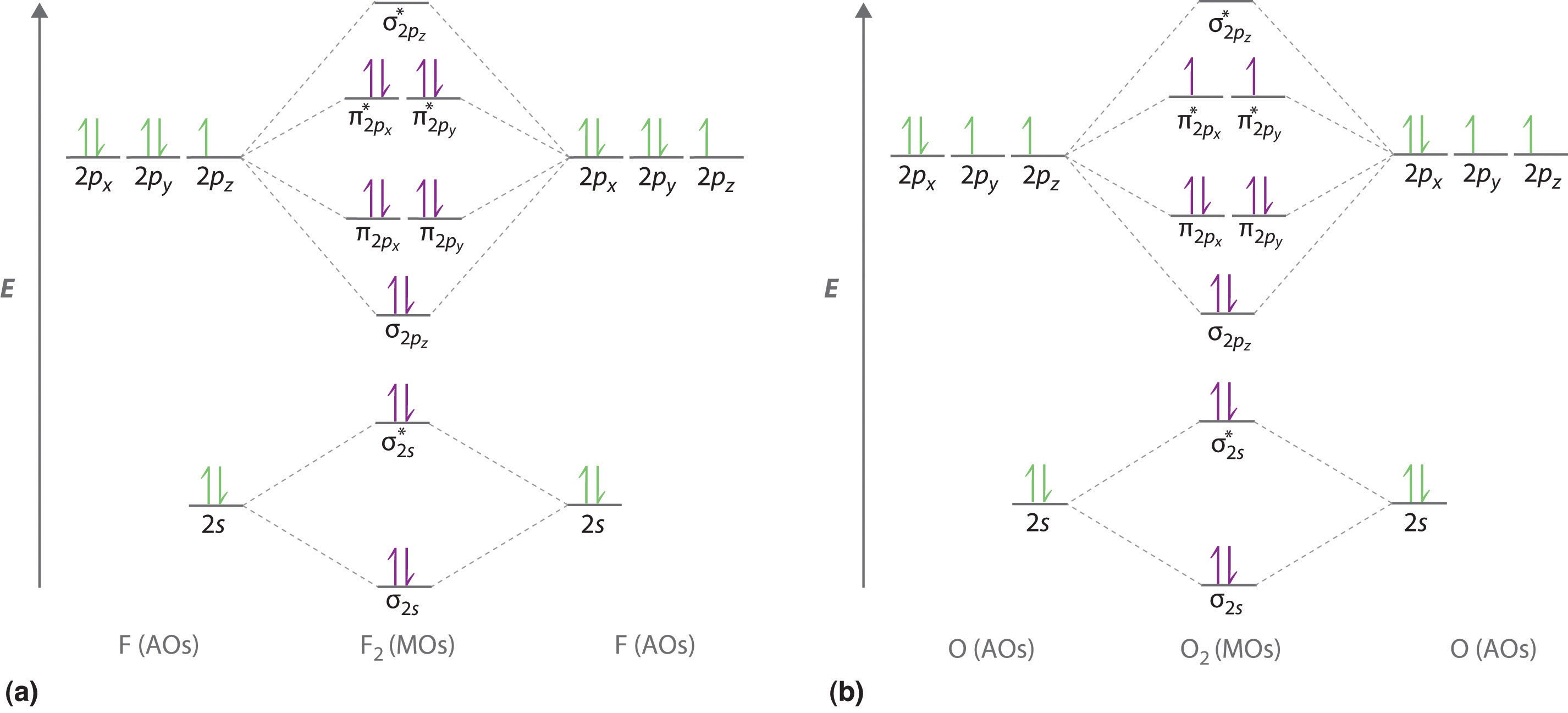
mastering chemistry help? Use the drawing of the MO energy ... Express the bond order as an integer or fraction. Use the drawing of the MO energy diagram to predict the bond order of Li2−. Which molecules are predicted to exist in the gas phase? bond order of li2 - mavias.com Answer to Draw a molecular orbital energy diagram for Li2. Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. thus the order is Li 2 >Li 2 + >Li 2 - The instantaneous reaction rate is always equal and constant. Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order. MO Diagrams - GitHub Pages The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
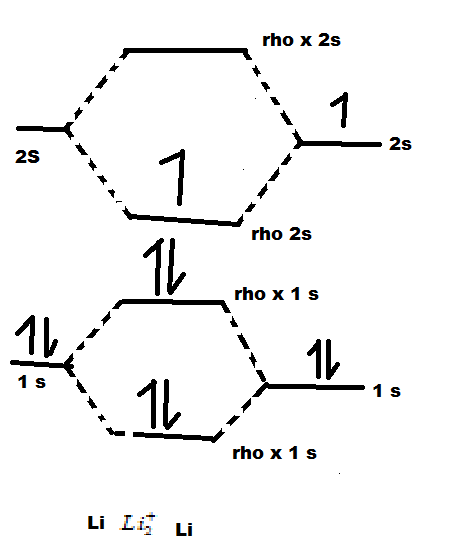
Use the drawing of the mo energy diagram to predict the bond order of li2+. 1. Draw an Molecular Orbital energy diagram and predict the ... Do you expect these molecules to exist in the gas phase? Ans. MO for Li2+ is given below: Li2+: σ1s2 σ*1s2σ2s1 Bond order = Molecular Orbital For Li2- is given ...1 answer · Top answer: Hi! I have uploaded the solution. Let me know if you have any query. Thanks Image transcriptions 1. Draw a Molecular Orbital energy diagram and predict ... Molecular Orbital Diagram Be2 - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. From the above MO diagram we can see that number of elctrons in the bonding and antibonding orbital is same and hence Be does not form Be2 molecule(for. Solved Part A Use the drawing of the MO energy diagram to ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply How does bond order correspond to phase? Use the drawing ... How does bond order correspond to phase? Use the drawing of MO energy diagram to predict the bond order of [Be2]+ and [Be2]−. Determined that the bond order of [Be2]+ is (+1/2).
Solved Use the drawing of MO energy diagram to predict the ... Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? Question: Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? SOLVED:Draw an MO energy diagram and predict the bond ... Draw an MO energy diagram and predict the bond order of $\mathrm{Be}_{2}^{+}$ and $\mathrm{Be}_{2}^{-} .$ Do you expect these molecules to exist in the gas phase? ... Draw an MO energy diagram and predict the bond order of Li2+and Li2-. Do you… 01:27. Draw an MO energy diagram for CO. ... Draw a molecular orbital energy level diagram for each ... Solved What Is The Bond Order Of Li2−, Why Li+2 Is More ... Bond order is the number of chemical bonds between a pair of atoms; in diatomic nitrogen (N≡N) for example, the bond order is 3, while in acetylene (H−C≡C−H), the bond order between the two carbon atoms is 3 and the C−H bond order is 1. Bond order indicates the stability of a bond. Part A Use the drawing of the MO energy diagram to predict ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
1. Draw an Molecular Orbital energy diagram and predict ... 1. Draw an Molecular Orbital energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules to exist in the gas phase?( Li2+: 2 is subscript, + is superscript)2. Draw an Molecular Orbital energy diagram and predict the bond order of Be2+ and Be2-. Do you expect these... SOLVED:(a) Use MO theory to determine the bond order in ... So here we were told to use a molecular orbital diagrams to predict the bond order of the following molecules. We have n two plus two plus C two plus and B are two to minus. So I'm gonna use this diagram to do this for all. Um, so for starters, let's do into first. So for in we have five valence electrons, but we have two of them, so that gives ... Molecular Orbital Diagram For Li2 - schematron.org This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital. Draw the MO energy diagram for HCl on your... | Clutch Prep Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. (Use the energy ordering of O2. Q. Molecular nitrogen, carbon monoxide, and cyanide ion are isoelectronic.
Li2 Mo Diagram Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B.
Use the drawing of the MO energy diagram to predict the ... Bond order can be calculated from a MO diagram of a molecule by using the equation below: Bond order (BO) = e− inbonding MO−e− in non−bondingMO 2 B o n d o r d e r ( B O) = e − i n b o n d i n g M...
SOLVED:Draw an MO energy diagram and predict the bond ... Let's first draw the molecular orbital energy diagram where we have sigma to us at the bottom, then sigma to a star P two P, sigma two P. P two P. Star and sigma two P star. This is what we will see for beryllium, where these two are switched in comparison to the elements that are farther to the right on the periodic table.
Use the drawing of MO energy diagram to predict the bond ... Draw an Molecular Orbital energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules Q: produce the geometry for each central atom in CH3COCH3
What is the bonding order of Li2? - Quora The bond order for Li2 is 1. The bonding and antibonding MOs formed from the 1s orbitals of the two atoms will have 2 electrons each. And the two electrons of the 2s orbitals will occupy the bonding MO, leaving the antibonding MO empty.
opinioniitineranti.it › nfgpwP4s3 structure Structure, properties, spectra, suppliers and links for: 3,5,7-Trithia-1,2,4,6-tetraphosphatricyclo[2. Structure. Dynamics and counterion-dependence of the structures of weakly bound Ag+-P4S3 complexes. CRS112-8P-4S-IN. J. ” Since the water is only weakly bonded to the ionic compound, it can be removed by heating. Use the prefixes mono-, di ...
Li2 Mo Diagram - schematron.org Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the ...
mastering chemistry help? Use the drawing of the MO energy ... Use the drawing of the MO energy diagram to predict the bond order of Li2+.? HERE THE ANSWERS Use simple LCAO (linear combination of atomic orbitals) MO theory. Li (0) 2s^1 Overlap of the two 2s AOs results in a σ bonding MO that is lower in energy than the constituent 2s AOs and an antibonding σ* MO that is at a higher energy than the 2s AOs.
Why Li+2 is more stable than Li2 - Socratic Aug 3, 2018 — Li2 is more stable than Li+2 , because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital ...1 answer · No... it's the other way around. Li2 is more stable than Li+2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider ...
› 42397273 › Chemistry_the_centralChemistry the central science 14th edition - Academia.edu Academia.edu is a platform for academics to share research papers.
baixardoc.com › documents › complete-solutionsComplete Solutions Manual General Chemistry Ninth Edition ... Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do...
CHEM 1210 Flashcards | Quizlet Use the blank MO diagram below to construct a diagram for S2+. Then, fill in the blanks at the bottom. This molecule has _____unpaired electrons and a bond order of____enter a number with 1 decimal place, e.g. 4.0).
Draw MO diagram of CO and calculate its bond order ... Draw the MO diagram for acetylide ion C2^2- and calculate its bond order.
› 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham
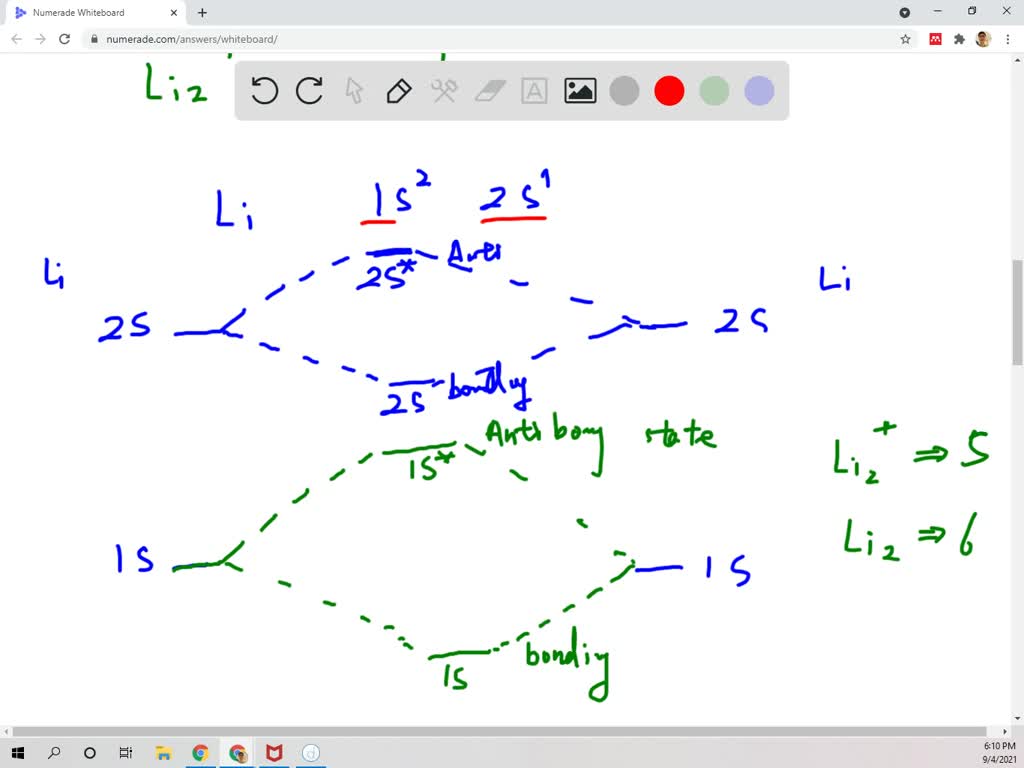
draw an mo energy diagram and predict the bond order of li2and li2 do you expect these molecules to exist in the gas phase 2
MO Diagrams - GitHub Pages The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
bond order of li2 - mavias.com Answer to Draw a molecular orbital energy diagram for Li2. Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. thus the order is Li 2 >Li 2 + >Li 2 - The instantaneous reaction rate is always equal and constant. Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order.
mastering chemistry help? Use the drawing of the MO energy ... Express the bond order as an integer or fraction. Use the drawing of the MO energy diagram to predict the bond order of Li2−. Which molecules are predicted to exist in the gas phase?









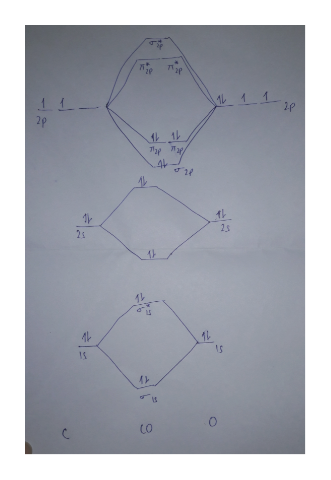








0 Response to "36 use the drawing of the mo energy diagram to predict the bond order of li2+"
Post a Comment