37 bromine lewis dot diagram
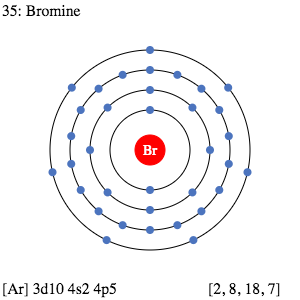
PDF Bohr Model Lewis dot diagram - WordPress.com Bohr Model Lewis dot diagram. 1. Determine the element's symbol 2. Determine the number of electrons 3. Determine number of valence electrons 4. Now draw your ... Bromine 79.904 Iodine 126.904 Astatine 209.987 117 Uus unknown VillA Helium 4.003 Neon 20.180 Argon 39.948 Krypton 84.80 Xenon 131.29 Radon 222.018 118 Uuo unknown Bromine - Home Therefore, if Bromine is in series 17, 17-10 is 7, so Bromine has 7 valence electrons. The electron dot diagram to the left depicts the number of valence electrons bromine has. The dots are arranged in a specific pattern to show how many valence electrons Bromine has.
Lewis dot diagram for bromine? - Answers Lewis dot diagram for bromine. Wiki User. ∙ 2011-06-14 04:21:10. Study now. See answer (1) Best Answer. Copy... Br :.. Wiki User. ∙ 2011-06-14 04:21:10. This answer is:
Bromine lewis dot diagram
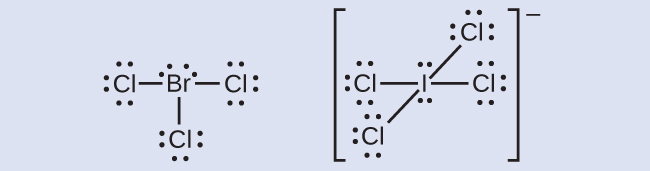
What is the Lewis dot structure for BrCl? 2022 - Question ... What is the Lewis dot structure for BrCl? In the BrCl3 Lewis structure Bromine (Br) is the least electronegative atom and goes in the center of the Lewis structure. For the BrCl3 Lewis structure, you'll need to put more than eight valence electrons on the Bromine atom. In the Lewis structure for BrCl3 there are a total of 28 valence electrons. Bromine Bohr Model - How to draw Bohr diagram for Bromine ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Bromine, we got to know, it has 7 valence electrons. So, just represent the 7 valence electron around the Bromine atom as a dot. Lewis Structures: Learn How to Draw Lewis Structures ... Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1.
Bromine lewis dot diagram. How to draw SBr2 Lewis Structure? - Science Education and ... To sketch the SBr2 Lewis structure by following these instructions: Step-1: SBr2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SBr2 for counting valence electrons around the terminal bromine atoms. Step-3: Lewis dot Structure for SBr2 generated from step-1 and step-2. Bromate ion (BrO3-) lewis dot structure, molecular ... Follow some steps for drawing the lewis dot structure of BrO3-1. Count total valence electron in BrO3-Lewis diagram is a simple representation of valence electron within a molecule. So, for determining the valence electron in BrO3-, look at the periodic group of bromine and oxygen atoms. (15) Lewis Diagrams (15) Lewis Diagrams Obj. 15. From the name of a molecular chemical, determine the Lewis (electron dot) diagram for it.. In these cases, you know that you are dealing with nonmetal atoms bonded together with covalent bonding and that (some of) the valence electrons of the atoms are shared between the atoms. How to Draw the Lewis Dot Structure for Br ( the ... - YouTube A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron...
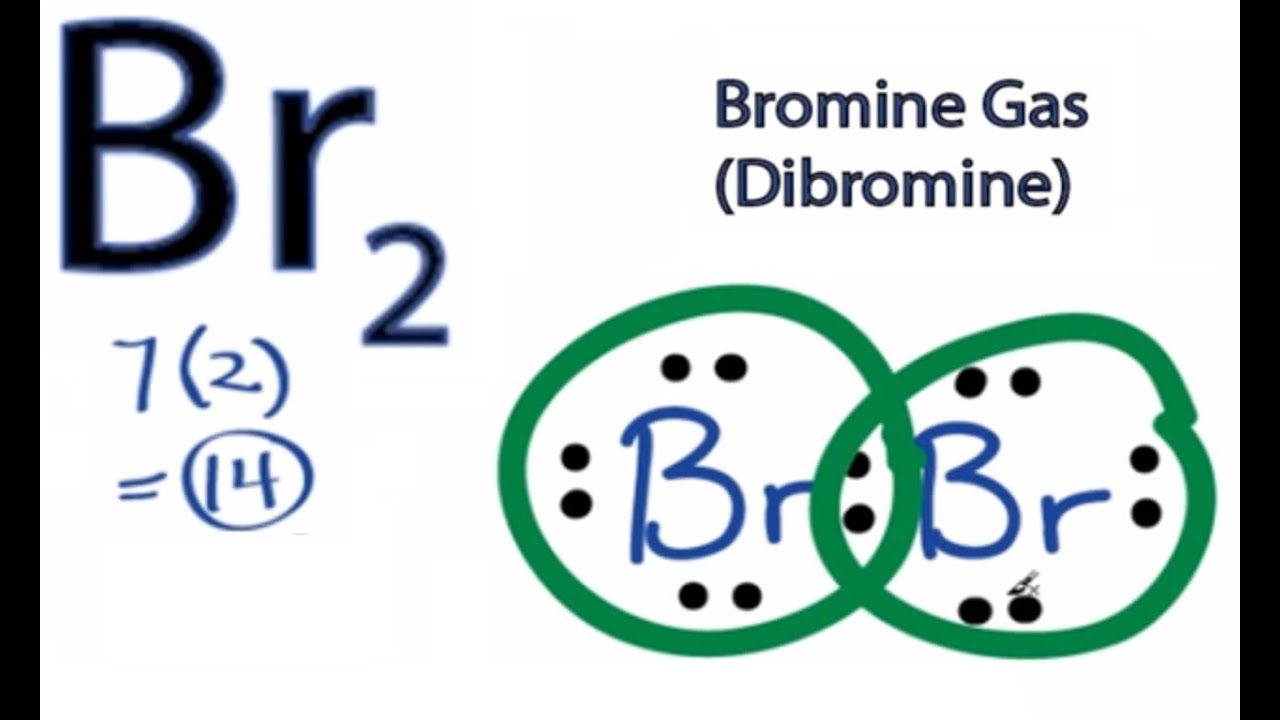
How to draw KBr Lewis Structure? - Science Education and ... KBr Lewis structure diagram, we always begin by introducing valence electrons from the central bromine atom(in step 2). As a result, wrap around the central bromine atom's bond pair valence electrons first (see figure for step2). The bromine atom in the molecule gets only 8 electrons around its molecular structure. Bromine (Br2) Lewis Structure - chemistryscl.com Lewis structure of bromine contains only one Br-Br bond and each bromine atom has three lone pairs. It is very easy to Br 2 lewis structure. Br 2 lewis structure. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. So, this lewis structure is a very simple. Steps of drawing lewis structure of Br 2 Lewis Dot Diagrams (Structures) for Atoms and Ions ... Lewis Dot Diagrams (Structures) for Atoms and Ions Predicting Oxidation Numbers. Pre AP Chemistry Unit 6 HW Packet Name _____ WKS 6.1- Classifying Ionic versus Covalent/ Lewis Dot Structures of Atoms. Classify the following compounds as ionic ([metal or ammonium ion] + [non-metal or polyatomic ion]), covalent (nonmetal+ nonmetal). CaCl2 / CO2 / H2O Lewis Dot Diagram - Organic Chemistry | Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
Br2 Lewis Structure, Molecular Geometry, Hybridization ... While the Lewis Structure provides an idea about the physical attributes of the compound, its representation is limited since it is a 2-dimensional model. It also does not reflect upon the molecular design, geometry, or the 3-dimensional representation of atoms. below are the steps to draw the lewis diagram of the Br2 molecule. BrF3 Lewis Structure, Molecular Geometry, Hybridization ... BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram BrF3, known as Bromine Trifluoride, is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. Having a straw i.e, colorless to yellow appearance, this chemical compound has several applications but also comes with a number of limitations and hazard issues. Boron tribromide (BBr3) lewis dot structure, molecular ... Boron tribromide (BBr3) lewis dot structure, molecular geometry, polar or nonpolar, hybridization Home > Chemistry Article > BBr3 lewis structure and its molecular geometry Boron tribromide composed of boron and bromine appears as colorless to amber liquid, has a sharp and irritating odor with chemical formula BBr3. How to Draw the Lewis Dot Structure for Br- (Bromide ion ... A step-by-step explanation of how to draw the Br- Lewis Dot Structure.For the Br- structure use the periodic table to find the total number of valence electr...
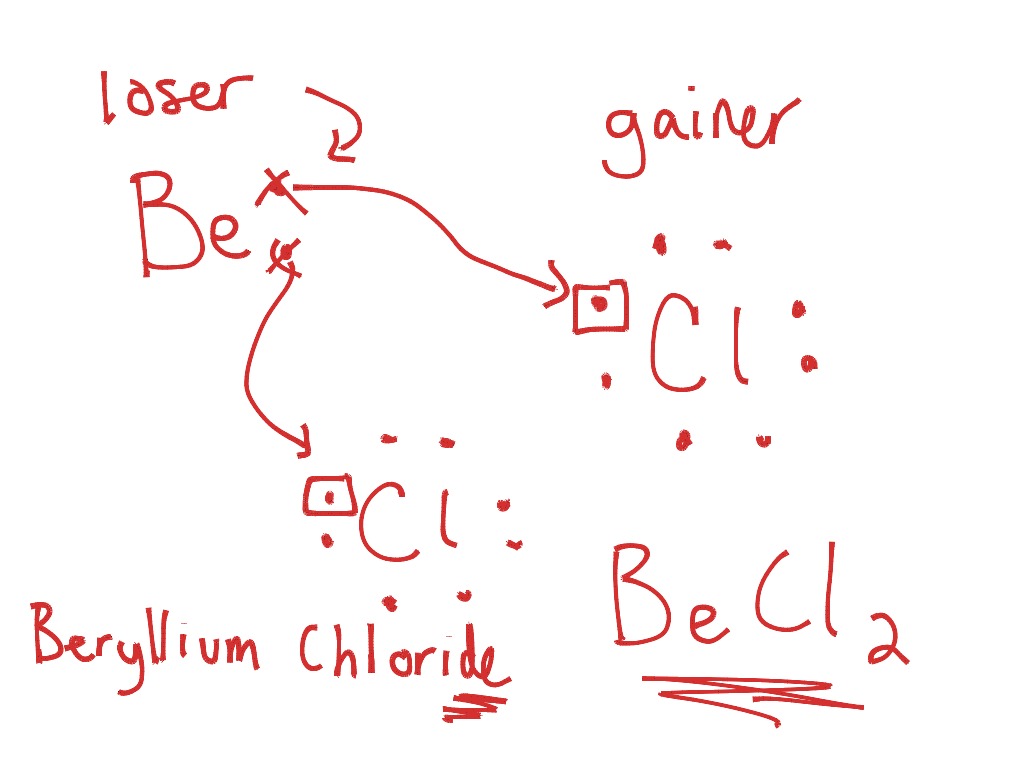
How would you represent potassium and bromine using an ... Here's how that would look. I'm not really sure if you're interested in the electron dot diagram of the potassium and bromine atoms, or of potassium bromide, "KBr", so I'll show you both. You can use this example to find the electron dot diagram of hydrogen bromide, "HBr". In order to draw an atom's electron dot diagram, you need to know two things the atom's chemical symbol the number of ...
Aluminum And Bromine Lewis Dot Structure - Novocom.top lewis diagram dot structure bromine pcl5 draw structures expanded octet brf5 dots drawing . bromine dot lewis electron structure symbol facts diagram interesting periodic properties table weebly . dot aluminum chloride electron formation transfer structure help brainiest plz helps hope mark .
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. ... Draw the Lewis electron dot diagram for each element. a) bromine. b) gallium. 9.
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Carbon tetrabromide (CBr4) lewis dot structure, molecular ... Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4. Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis diagram. "A valence electron is the outermost shell electrons around an atom".
=章 9 Section A Lewis Electron Dot Diagrams For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4 s 2 3 d 6 ) is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Br2 Lewis Structure - How to Draw the Lewis Dot ... - YouTube A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Bromine gas).For the Br2 structure use the periodic table to find the total number of ...
Lewis dot diagram for bromine? - Answers Lewis dot diagram for bromine? Asked By Wiki User. Unanswered Questions . Is it advantageous or disadvantageous to use one pipette throughout the dilution process and why? Asked By ...
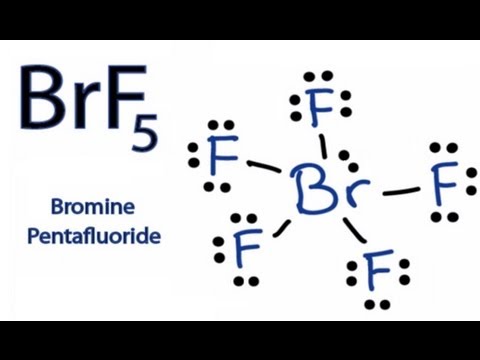
Bromine pentafluoride (BrF5) lewis dot ... - Topblogtenz Bromine pentafluoride is polar in nature. The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral. BrF5 lewis dot structure has 10 sharing electrons and 32 non-sharing electrons. The bond angle of BrF5 is 90º.
How to Draw the Lewis Dot Structure for NaBr: Sodium bromide A step-by-step explanation of how to draw the NaBr Lewis Dot Structure.For NaBr we have an ionic compound and we need to take that into account when we draw ...
Lewis Structures: Learn How to Draw Lewis Structures ... Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1.
Bromine Bohr Model - How to draw Bohr diagram for Bromine ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Bromine, we got to know, it has 7 valence electrons. So, just represent the 7 valence electron around the Bromine atom as a dot.
What is the Lewis dot structure for BrCl? 2022 - Question ... What is the Lewis dot structure for BrCl? In the BrCl3 Lewis structure Bromine (Br) is the least electronegative atom and goes in the center of the Lewis structure. For the BrCl3 Lewis structure, you'll need to put more than eight valence electrons on the Bromine atom. In the Lewis structure for BrCl3 there are a total of 28 valence electrons.

![Answered] Add electron dots and charges as necessary to show ...](https://us-static.z-dn.net/files/d4b/88841f75d215cbd3475d7a4ced0e6af5.png)

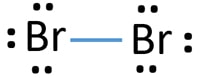

















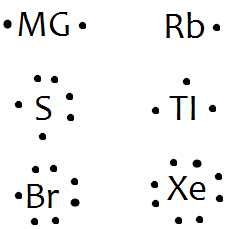





0 Response to "37 bromine lewis dot diagram"
Post a Comment