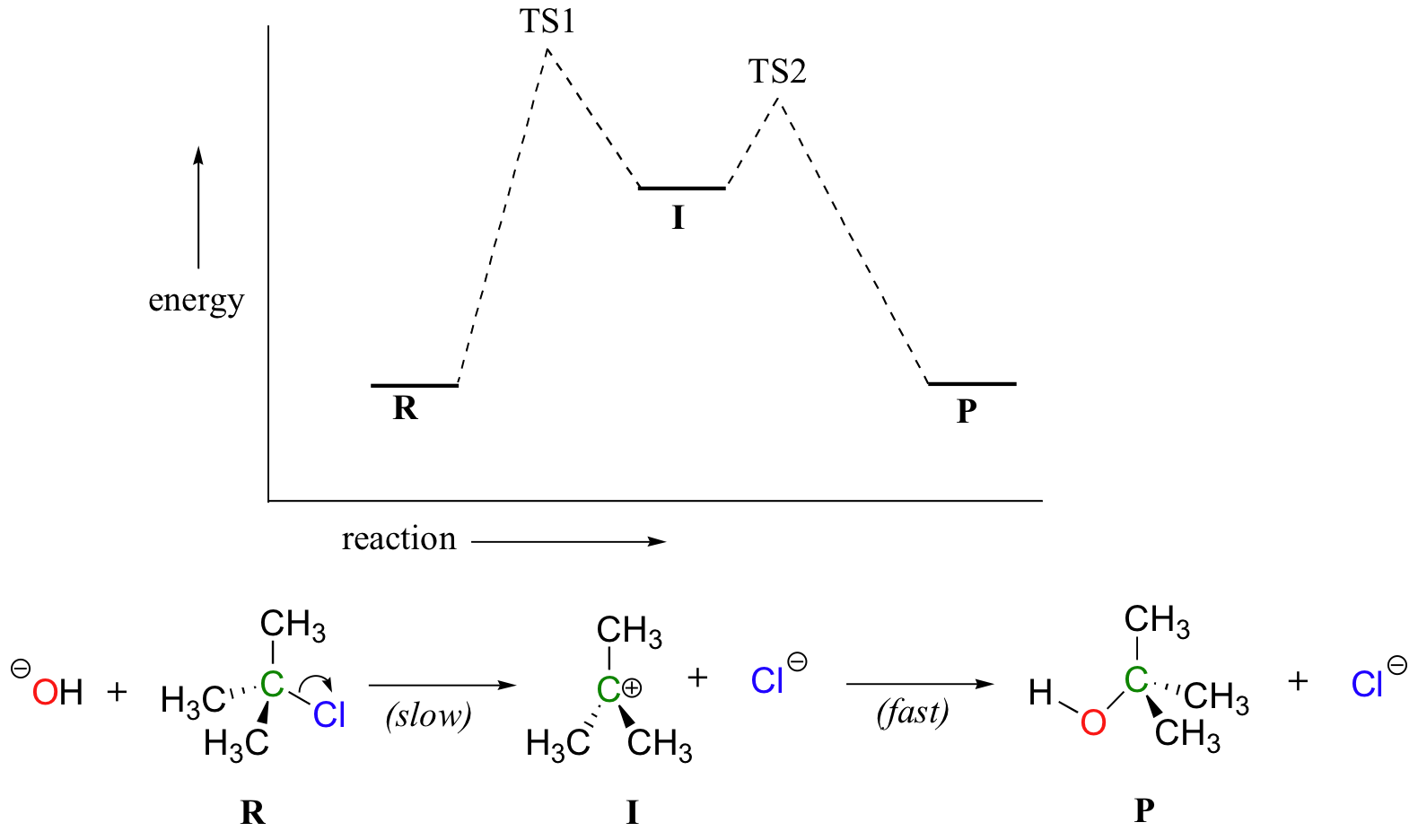
38 how is activation energy represented on an energy diagram
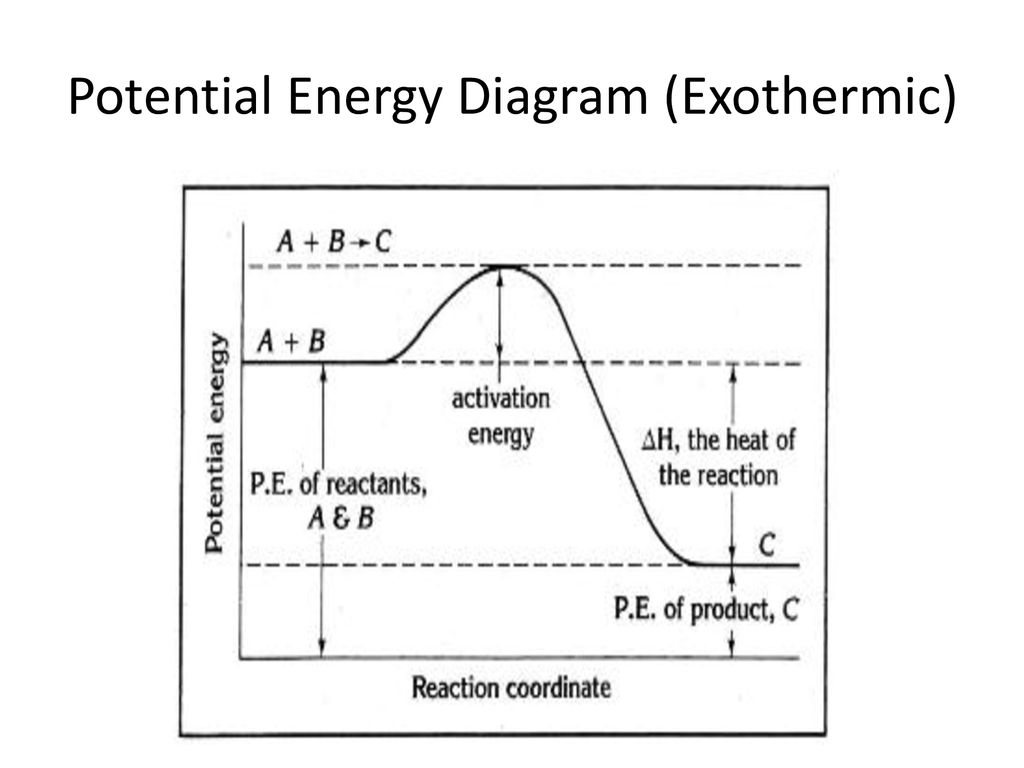
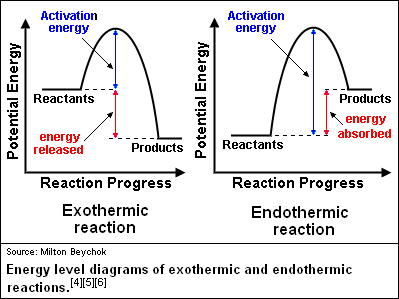
Energy Changes in Chemical and Physical Processes ... Activation Energy Is the energy required to activate the reactants before a reaction can take place. Thus activation energy is the energy required to initiate a reaction. The size of activation energy will differ from one reaction to another and so will be the gap between the energy of reactants and the energy is products. Examples: 36 the diagram represents a reaction. use the diagram to ... What is the activation energy of the reaction?25 Apr 20181 answer · Top answer: a) exothermic; b) 25 kJ The diagram represents a reaction. use the diagram to answer the questions below. William L. Masterton, Cecile N. Hurley, Edward Neth · 2011 · Science (a) Write the equation for the reaction represented by the diagram. ...
Energy Diagrams Video & Text Solutions For College ... Access 145 Energy Diagrams video and text solutions to help you complete your homework. ... Calculate the activation energy, ΔG for the step C to B _____ kcal/mol Cal... Solved • Nov 25, 2020 ... On a reaction energy profile diagram, how is an intermediate represented? Solved • Dec 6, 2018

How is activation energy represented on an energy diagram
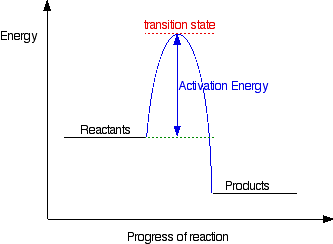
The diagram shows the potential energy changes for a ... The activation energy ( E (B)-E (A)) is positive as well. We have a diagram showing the potential energy changes for a reaction pathway. Point A represents the potential energy of the reactants. Point B represents the potential energy of the transition state. Point C represents the potential energy of the products. (Get Answer) - What does a catalyst do for a reaction ... The rate of a reaction can be increased by using a catalyst. A catalyst provides an alternative reaction pathway that has a lower activation energy. Energy profile diagrams (like the one to the right), which can represent catalysed and uncatalysed... The activation energies of the reaction represented by the ... The activation energies of the reaction represented by the following reaction energy diagram are noted as I and II.
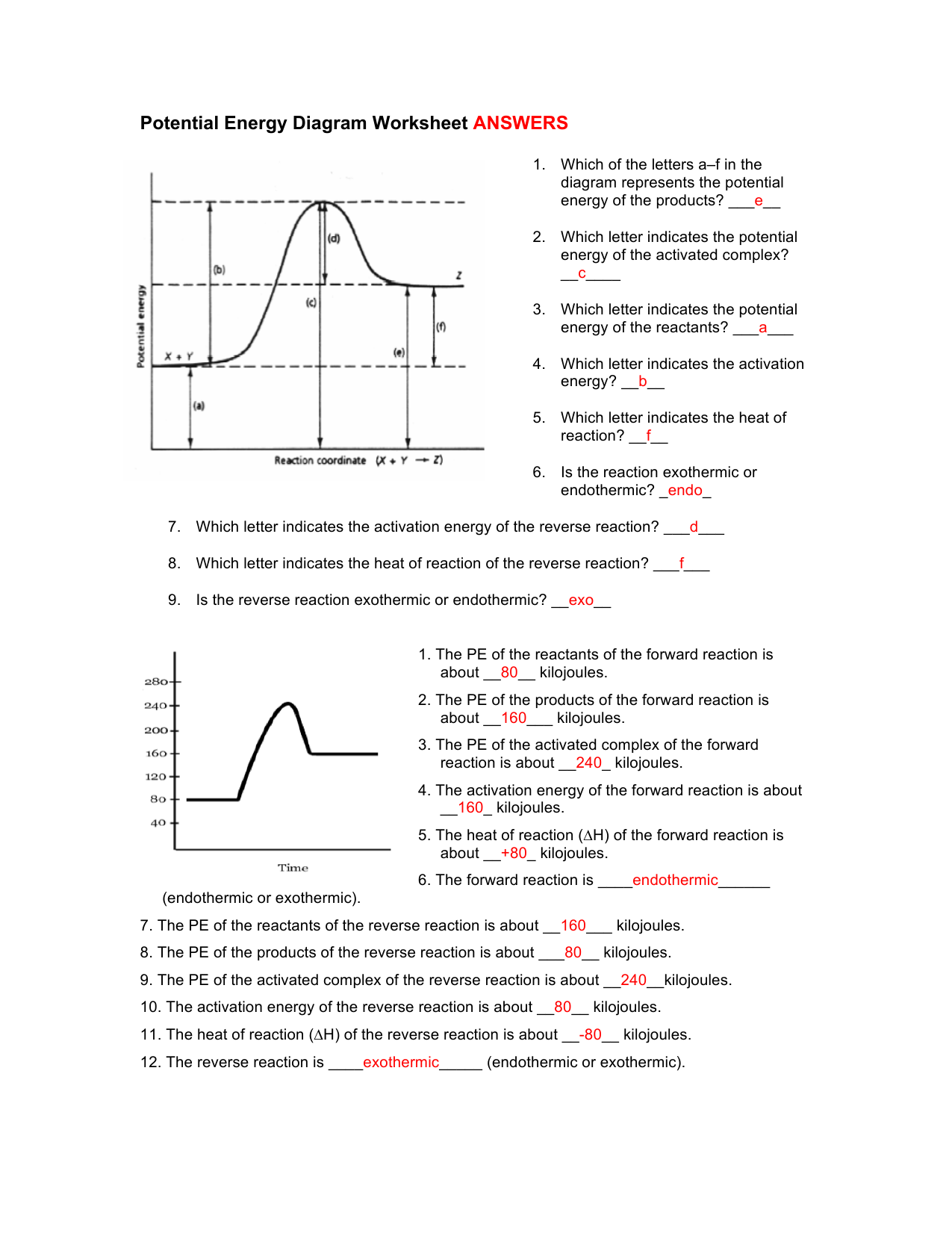
How is activation energy represented on an energy diagram. Glycolysis : All Steps with Diagram, Enzymes, Products ... The energy-requiring phase (Preparatory phase) The energy-releasing phase. (Payoff phase) Glucose-requiring phase (Preparatory Phase) This phase is also called the glucose activation phase. In this phase of glycolysis, two molecules of ATP are invested, and the hexose chain is cleaved into two triose phosphates. It is time to practice using potential energy diagrams ... In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ. Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction. The diagram shows the potential energy changes for a ... The diagram shows the potential energy changes for a reaction pathway. A curved lined graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The graph curve begins at a lower level and ends at a slightly higher level. Understanding potential-dependent competition between ... We constructed the potential-dependent free energy diagram for NRR at 0, −0.23, and −0.5 V (vs. RHE at pH = 13) including activation energies by using the CEP model (Fig. 3). Calculation details for obtaining reaction energy and activation free energy under constant potential are shown in the "Methods" section and Supplementary Note 2.
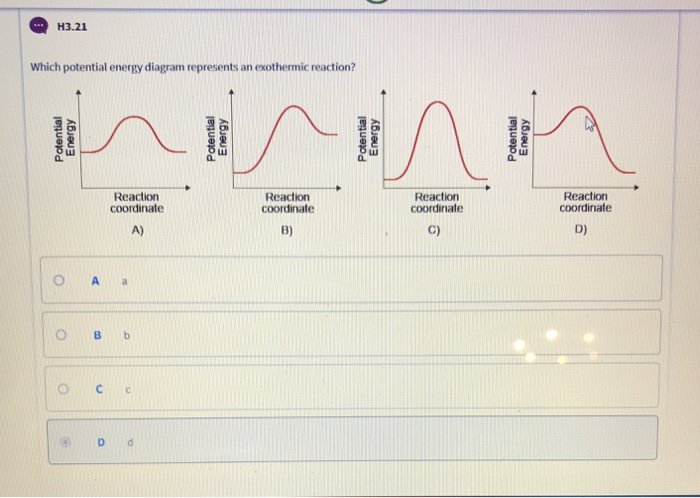
TUTORIAL CHEMISTRY SK025 - Flip eBook Pages 1-50 | AnyFlip (b) Draw and label the potential energy profile for the reaction with and without TUTORIAL 8.0 REACTION KINETICS platinum. 8. (a) What is meant by activation energy? (b) The rate constant of a reaction at 463 K is 2.52 10−5 s−1 and at 503 K is 6.30 10−4 s−1. Determine the activation energy for the reaction. (155.646 kJ mol−1) Chemistry 07.01 Endothermic and Exothermic ... - Quizlet Compare the slope of the line on a potential energy diagram for an endothermic reaction and an exothermic reaction. Due to the decrease in enthalpy between reactants and products, potential energy diagrams for endothermic reactions have a positive, or upward, slope that represents a +q or + H. Co-thermal degradation characteristics of rice straw and ... Co-thermal treatment of binary biomass mixture is an alternative to enhance the refractory decomposition of biomass thermal degradation efficiency resulted in the synergistic reaction. Rice straw (RS) containing a large amount of fixed carbon (FC) is quite difficult to thermally decompose at a lower temperature. Considering the RS and sewage sludge (SS), co-thermal treatment for enhancing ... Classroom Resources | Energy Changes in Chemical Reactions ... In this simulation, students will evaluate the energy changes in an endothermic and an exothermic chemical reaction. Students will have the opportunity to compare how energy is absorbed and released in each reaction, and will make a connection between the standard energy diagrams associated with each reaction type.
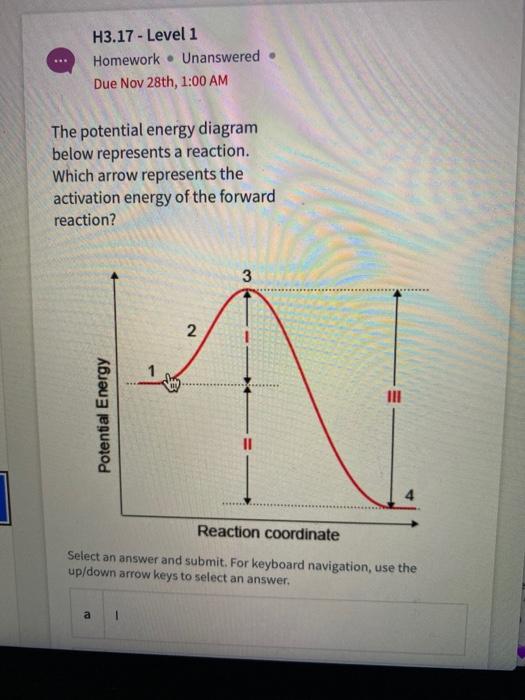
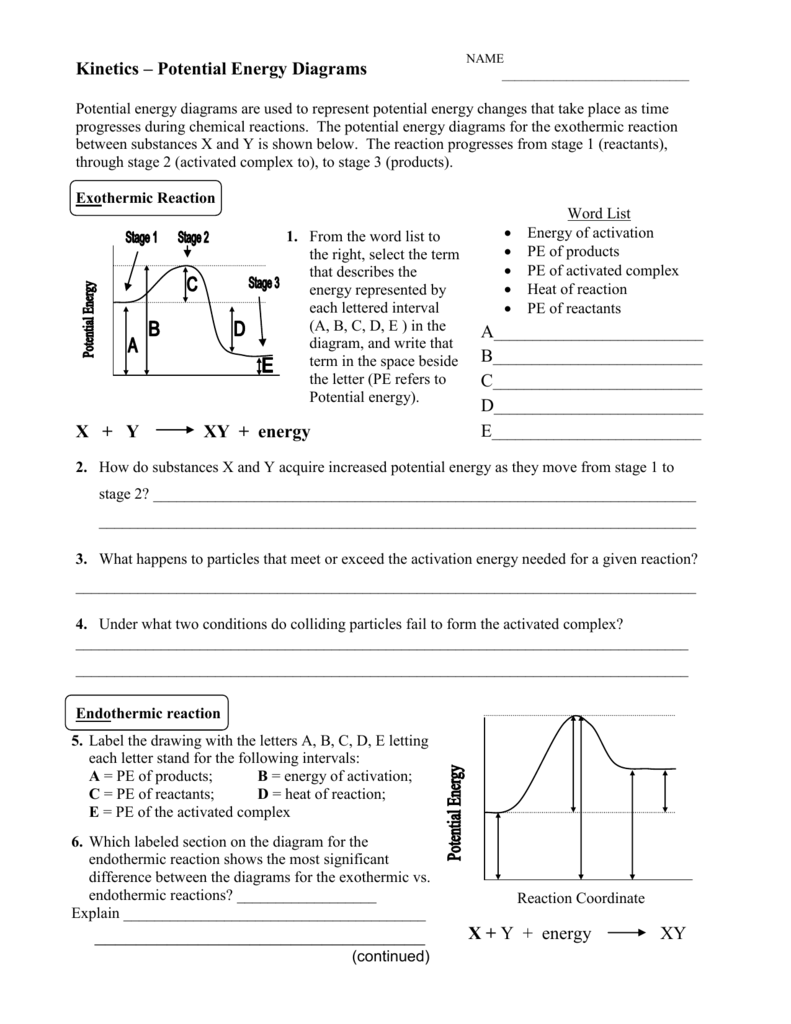
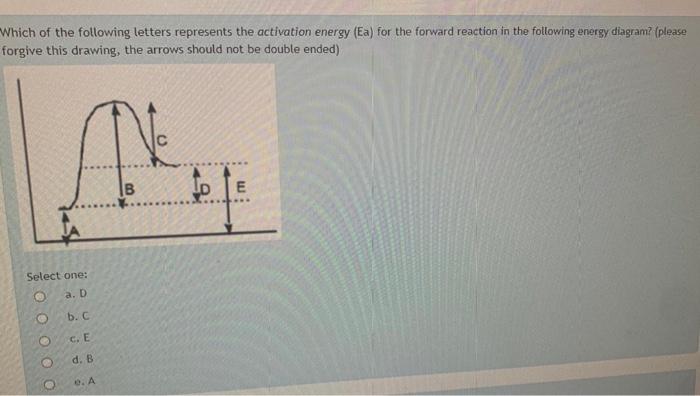
Looking at a potential energy diagram what is the name ... 17 March 2022 by Huwas Looking at a potential energy diagram what is the name given to represent the amount of energy needed for the Looking at a potential energy diagram what is the name given to represent the amount of energy needed for the reaction to take place? Activation energy Free energy Stored energy Released energy On the energy diagram below, which arrows) represent the ... On the energy diagram below, which arrows) represent the activation energy for the forward and reverse reactions? A) Arrow 1 is the activation energy for both the forward and reverse reactions. B) Arrow 1 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction. 41 energy in reactions worksheet - Worksheet Master The mathematical formula for this value is: ∆G = ∆H - T∆S Where ∆H = change in enthalpy or heat of reaction, T = temperature in Kelvin, ∆S = change in entropy DOC Reaction Energy & Rates Worksheet Worksheet 11.1. Part A - Reaction Energy. Fill in the blanks on the reaction coordinate diagram with the appropriate letters. Understanding potential-dependent competition between ... Here, Ga (*N 2 → *NNH) (or ∆ G (*N 2 → *NNH)) represents activation energy (or reaction energy) of *N 2 + (H + + e− ) → *NNH. The potential-dependent free energy diagrams for *NNH formation are...
Activation Energy Activation energy, Ea = Threshold energy - Average kinetic energy of reacting molecules. In a graph of potential energy vs reaction coordinate, the reaction coordinate indicates the profile of energy shift as reactants transform into products in a graph of potential energy vs reaction coordinate.
CHAPTER 1.0 REACTION KINETICS_NOTES & TUTORIAL Q's - Flip ... 1.3 Factors Affecting b) Define activation energy. Reaction Rate c) Explain transition state theory. d) Draw energy profile diagram of a reaction. a) Explain the effect of the following factors on the reaction rate: i. concentration or pressure ii. temperature iii. catalyst iv. particle size. *Exclude most probable molecular and average kinetic ...
Computational Study on Three-Dimensional Convective Casson ... The activation energy has various focal points with respect to the design of chemical production, manufacturing of food items, geothermal repository, oil emulsion formation, etc. Activation energy investigations of a nano Casson fluid flow were carried out by Gireesha et al. with an exponential spatial source of thermal energy and a binary ...
Enzymes Function & Structure | What is the Role of Enzymes ... Enzymes represent special proteins that enable reactions to occur at faster rates. Enzymes are able to increase the speed of reactions by lowering the activation energy of the reaction. The ...
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... First, as noted, the y -axis is labeled 'enthalpy' and the x -axis is labeled 'reaction progress.' Then we have the actual energy diagram plot. Typically the energy first goes up, and this is the...
equal and opposite energy quick check A researcher can ... Which is conduction?(1 point) A.) energy transfer from a reaction system to its surroundings B.) energy transfer as electromagnetic waves C.) energy transfer by currents of moving liquid or gas D.) energy transfer by direct . Chemistry. The diagram represents the reaction of hydrogen gas and oxygen gas to produce water.
Federal Register :: Energy Conservation Program: Test ... The U.S. Department of Energy (``DOE'') proposes to amend the test procedure for variable refrigerant flow multi-split air conditioners and heat pumps (``VRF multi-split systems'') to incorporate by reference the latest version of the industry test standard. DOE also proposes to adopt the...
Molecular-level insights into the electronic effects in ... a Schematic diagram of CNT activations via covalent functionalization and heat treatment.
Interactions among mTORC, AMPK and SIRT: a computational ... A more comprehensive diagram can be found in Additional file 2: Fig. S1, where the activated and inactivated forms of a protein are represented separately, together with their individual interactions, to depict the pathways and protein interactions.
Activated Sintering - Powder Metallurgy - Beyond Discovery In radiation bombardment, sintering kinetics are altered by the creation of a vacancy excess. This eliminates the vacancy formation energy from the activation energy for diffusion. Alternatively, treatments such as cyclic heating of a material such as iron through the polymorphic phase transformation represent a change in the driving force.
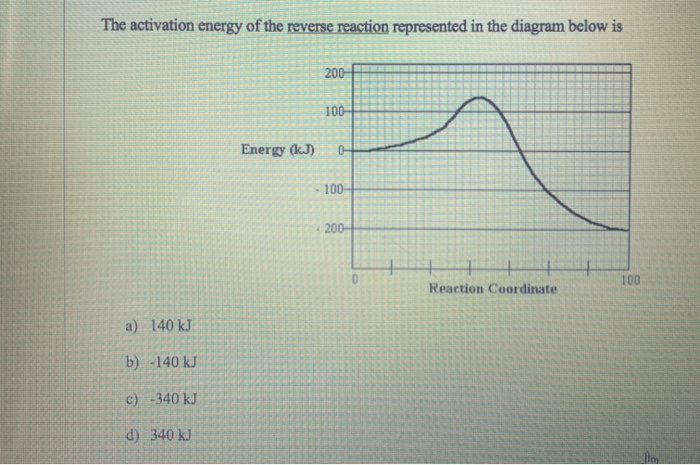
PLSSSSS HELPPPPP Consider the potential energy diagram ... Explain your answer in two ways: first, using the energy values, and second, by referring to the shape of the graph. The coating on the head of a match is highly flammable. When it burns, it releases a great deal of energy. However, before the match can burn, it must gain a small amount of energy from a spark.
The activation energies of the reaction represented by the ... The activation energies of the reaction represented by the following reaction energy diagram are noted as I and II.
(Get Answer) - What does a catalyst do for a reaction ... The rate of a reaction can be increased by using a catalyst. A catalyst provides an alternative reaction pathway that has a lower activation energy. Energy profile diagrams (like the one to the right), which can represent catalysed and uncatalysed...
The diagram shows the potential energy changes for a ... The activation energy ( E (B)-E (A)) is positive as well. We have a diagram showing the potential energy changes for a reaction pathway. Point A represents the potential energy of the reactants. Point B represents the potential energy of the transition state. Point C represents the potential energy of the products.





















0 Response to "38 how is activation energy represented on an energy diagram"
Post a Comment