38 lewis dot diagram for n2
Answered: Draw the Lewis Dot Structure for N2.… | bartleby Homework help starts here! Browse 5+ million homework and textbook solutions, concept explainers, videos and more. Search concepts or drop in your homework problem! Our library grows every minute-keep searching! Science Chemistry Q&A Library Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or ... N2 Lewis Structure | Lewis Structure N2 | HND Assignment How is n2 formed in n2 lewis structure? The Lewis Structure or the Lewis Dot Structure or the Lewis Dot Diagram, named after Gilbert N. Lewis, shows the diagram of the atomic bonding of the molecules or an element. It shows the lone pairs of molecules existing in a molecule.
Lewis Dot Diagram For So4 2 - Wiring Diagrams Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
Lewis dot diagram for n2
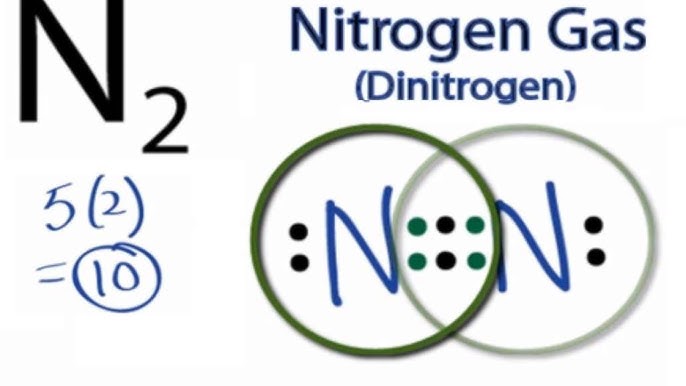
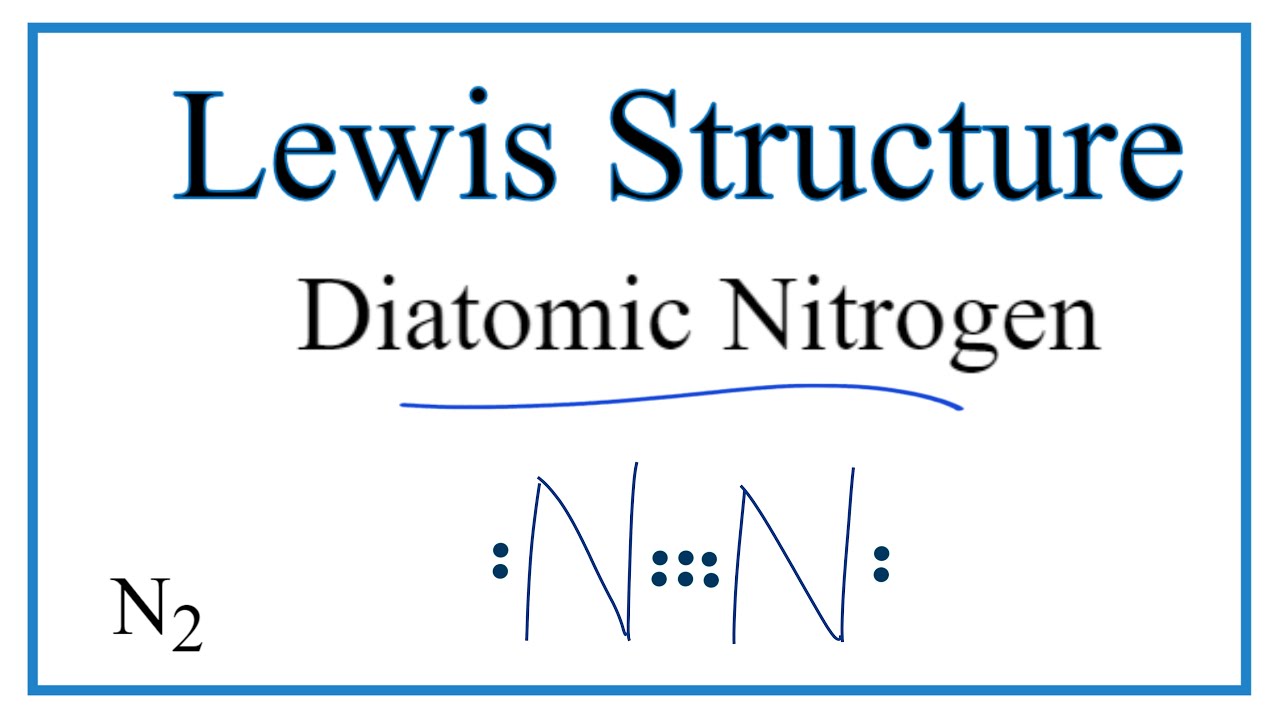
How to do lewis dot structure (2022) - VerseDaily N2 Lewis structure there is a triple connection between the two nitrogen atoms. According to the octet rule, nitrogen the atoms must be connected three times. The N2 molecule is diatomic, which means that two atoms of the same element are paired. Answered: Draw the Lewis Dot structure for N2 (on… | bartleby Science Chemistry Q&A Library Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express your answer as a whole number. b. How many single bonds are in N2? Express your answer as a whole number. Lewis Dot Diagram For N2 - schematron.org If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like? N (triple bond)N and then two dots on each N in which ever spot is open. Share to.Lewis structure - WikipediaWhat is the Lewis structure of N2
Lewis dot diagram for n2. images.pcmac.org › SiSFiles › SchoolsAP Chemistry Lab Manual - PC\|MAC A smooth line should be drawn that lies as close as possible to most of the points. Do NOT draw a line connecting one point to the next as in a dot-to-dot drawing. If the line is a straight line, use a ruler to draw it. If a computer program is used to draw the graph, the rules still apply. Accuracy What is the Lewis structure of N2? - Answers What is the Lewis dot formation of N2? The N2 molecule consists of two nitrogen atoms held together by a triple bond. Each nitrogen atom also has a lone-pair of electrons. Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below: N2 Lewis Structure: Full Guide (2022 Updated) N2 Lewis Structure: What You Need To Know. The Lewis structure of the N2 would consist of two Nitrogen atoms connected by a triple bond. The octet rule states that nitrogen atoms must link three times. The N2 molecule is diatomic, Each Nitrogen atom has one lone pair of electrons. Nitrogen has five valence electrons in the N2 electron dot ...
Draw electron dot structure of CO2,H2O,F2,N2 Draw electron dot structure of CO 2 ,H 2 O,F 2 ,N 2 . Draw electron dot structure of. C. Draw the Lewis structure for N2. Nitrogen is an unreactive ... The lewis structure of n2h2 shows c. each nitrogen has one nonbinding electron pair Lewis structures are diagrams that show the bonds between the atoms of a molecule and the lone pair of electrons that might exist in a molecule. Further Explanation Lewis structures can be drawn for each covalently bonded molecule, as well as coordination compounds. Lewis Structure of N2 (Nitrogen Gas) - YouTube How to Draw the Lewis Structure of N2 - with explanation!Check me out: N2 Lewis Structure| Hybridization & Molecular Geometry ... 5. Explain N2 dot structure in simplest form. N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in ...
quizlet.com › 195082504 › chem-flash-cardschem Flashcards - Quizlet The mass number is the number of dots in the Lewis dot structure for any element. The Lewis dot structure is used to keep track of the valence electrons for each atom. Give the symbol of the element in Group 6A, Period 3. N2 Lewis Structure - Easy Hard Science N2 Lewis Structure Setup It's easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won't bond, on top of each N. What is the Lewis dot structure for nitrogen gas ... Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the nitrogen atoms. What type of gas is nitrogen? N2 Lewis Structure, Molecular Geometry, and Hybridization ... Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion In the Lewis structure of the N2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
Solved 1. Draw lewis dot structures for O2 and N2 se the ... 1. Draw lewis dot structures for O2 and N2 se the density of dry air to calculate the mass, in grams, of a 240 mL sample of air at a pressure of 750 mmHg and a temperature of 19°C Refer to the chart in the lab techniques section for dry air density in units of E/L An unknown gas sample is collected in a 255 mL container at 25'C and 755 mmHg.
Nitrogen (N2) Molecule Lewis Structure Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's lewis structure.
Solved How to draw the Lewis structure for N2+ | Chegg.com Question: How to draw the Lewis structure for N2+ This problem has been solved! See the answer See the answer See the answer done loading. How to draw the Lewis structure for N2+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality ...
PDF Lewis dot structure of n2 molecule Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
What is the Lewis dot structure for N2? - Answers a Lewis dot structure. What is the Lewis dot structure for N2 look like? N(triple bond)N and then two dots on each N in which ever spot is open. Lewis dot structure for carbon? The Lewis Dot...
What is the Lewis Structure for N2 (nitrogen gas)? - Quora Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
N2 Lewis Structure - draw the electron dot structure of o2 ... N2 Lewis Structure. Here are a number of highest rated N2 Lewis Structure pictures on internet. We identified it from reliable source. Its submitted by organization in the best field. We agree to this kind of N2 Lewis Structure graphic could possibly be the most trending subject past we part it in google improvement or facebook.
What is the Lewis structure of N2? | Socratic In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
N2 Lewis Structure: How to Draw the Dot Structure for N2 ... Drawing the Lewis Structure for N 2 Viewing Notes: Make sure you count the number of valence electrons correctly. Nitrogen is in group 5A (also called Group 15). Each Nitrogen atom has five valence electrons. Since there are two Nitrogen atoms in N 2 you have a total of ten valence electrons to work with.
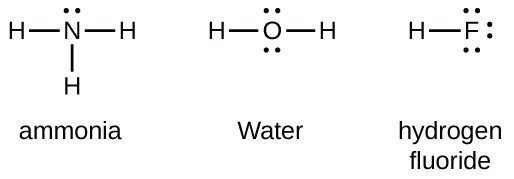
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
PPT PowerPoint Presentation - Chemical BONDING Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
Lewis Dot Structure For Nitrogen Disulfide - Novocom.top nitrogen dot lewis structure molecule n2 symbol atom electrons valence hydrogen atoms electron draw simple correct elements between polar properties . ... ammonia electron lone lewis dot diagram pairs pair nitrogen geometry molecular molecule formula atom ch4 electrons single chemistry bonds contains .
Lewis Dot Diagram For N2 - schematron.org If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like? N (triple bond)N and then two dots on each N in which ever spot is open. Share to.Lewis structure - WikipediaWhat is the Lewis structure of N2
Answered: Draw the Lewis Dot structure for N2 (on… | bartleby Science Chemistry Q&A Library Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express your answer as a whole number. b. How many single bonds are in N2? Express your answer as a whole number.
How to do lewis dot structure (2022) - VerseDaily N2 Lewis structure there is a triple connection between the two nitrogen atoms. According to the octet rule, nitrogen the atoms must be connected three times. The N2 molecule is diatomic, which means that two atoms of the same element are paired.









![Expert Answer] Draw the electron dot structure of nitrogen ...](https://hi-static.z-dn.net/files/da9/f9dadd9fdaba09b20251ec55355bd804.jpg)





![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1648865_1784763_ans_65460fbc5eda4a3b8021b75bbc2803a5.png)







0 Response to "38 lewis dot diagram for n2"
Post a Comment