38 o2 2- mo diagram
PDF MO-Schema O2 - uni-potsdam.de MO-Schema O 2-(Superoxid)Energie 2p 2s 1s 1s * 1s b 2p 1s 2s b 2s * 2s b p x b p z *p x. Title: PowerPoint-Präsentation Author: A.Kelling Created Date: 6/30/2011 9:20:45 AM Molecular Orbital Diagram For Cl2 The Lewis dot structure famously predicts the wrong electronic structure for O2. • We can use LCAO-MO theory to get a better picture: 2sa. 2pa.MO Diagram of I 2 MO Diagram of I 2-Base Complex In this experiment we analyze the acid-base interaction by comparing the energies of the I2 transition to that of the donor-acceptor transition.
o2 | Editable Organizational Chart Template on Creately o2 ( Organizational Chart) Use Creately's easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. We were unable to load the diagram. You can edit this template on Creately's Visual Workspace to get started quickly. Adapt it to suit your needs by changing text and adding colors ...

O2 2- mo diagram
N2+ Mo Diagram 2- = Molecular orbital for N2, N2+, O2, H2 and He2 by Thomas Wells - December 5, Brian Verfuerth 0. Ozone Lewis diagrams and by avatar Claire Bridget . The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. N2+ Mo Diagram - schematron.org There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. What's the MOT diagram of O2 +2 ion? - Quora In normal O2, there are 6 bonding electrons and 2 antibonding electrons, making the bond order 2. By removing the 2 highest electrons, which reside in ...3 answers · 9 votes: In O2 2+, there is 14 electrons. So, it’s MOT is comparable to N[code ]2[/code] & the MOT ...
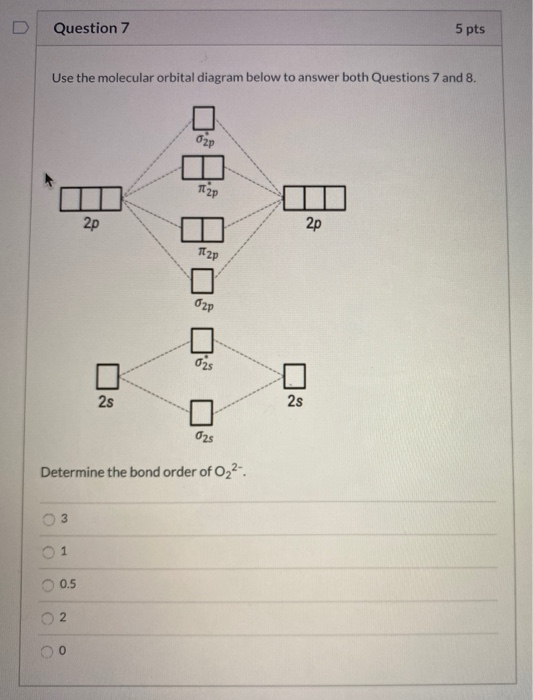
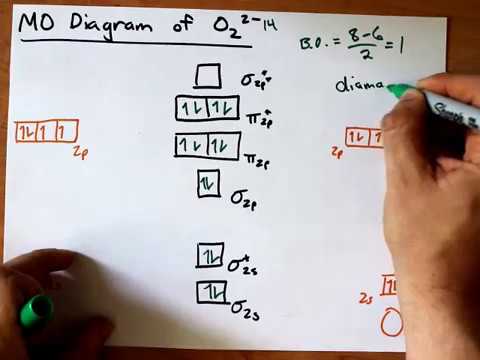
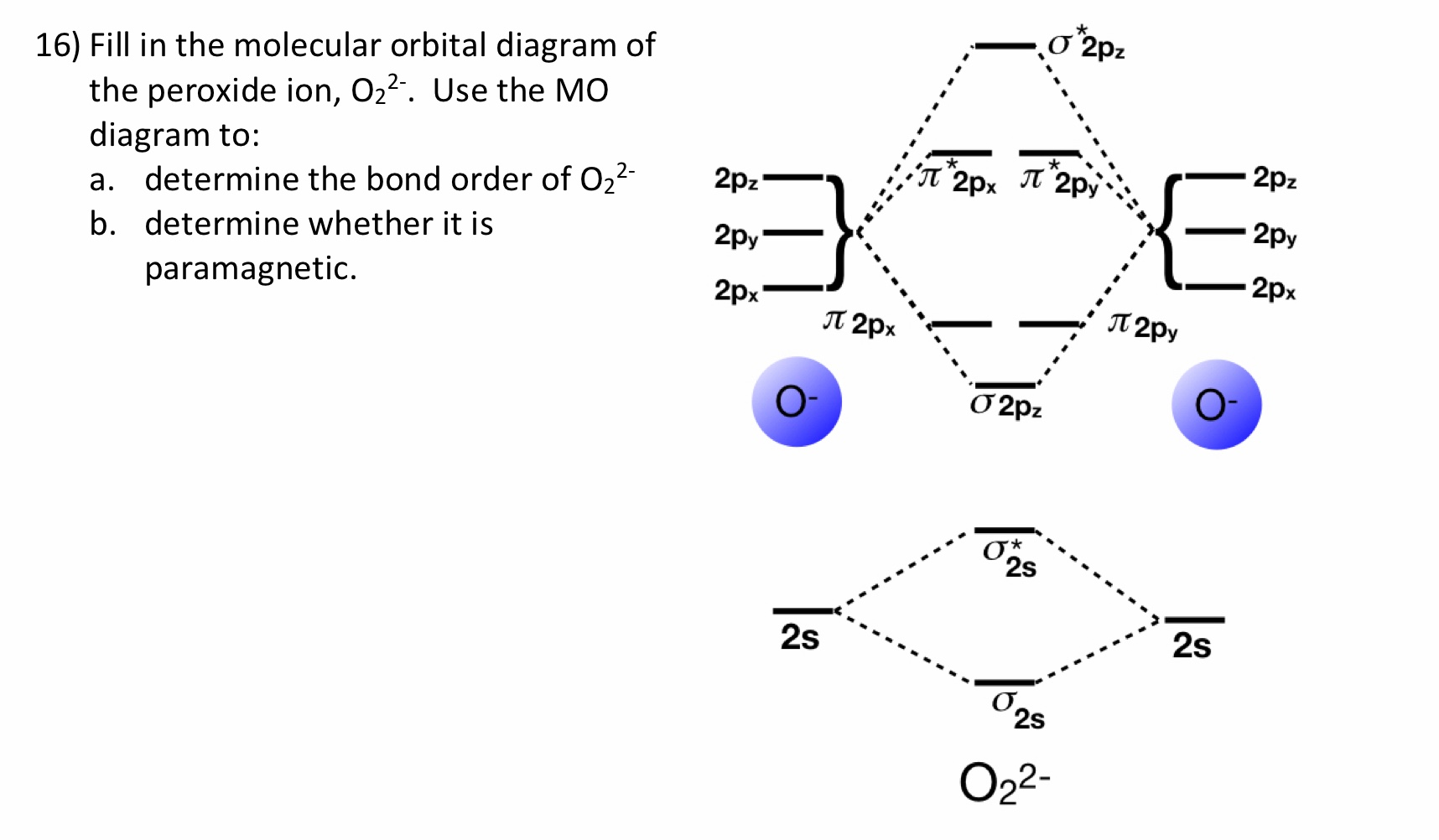
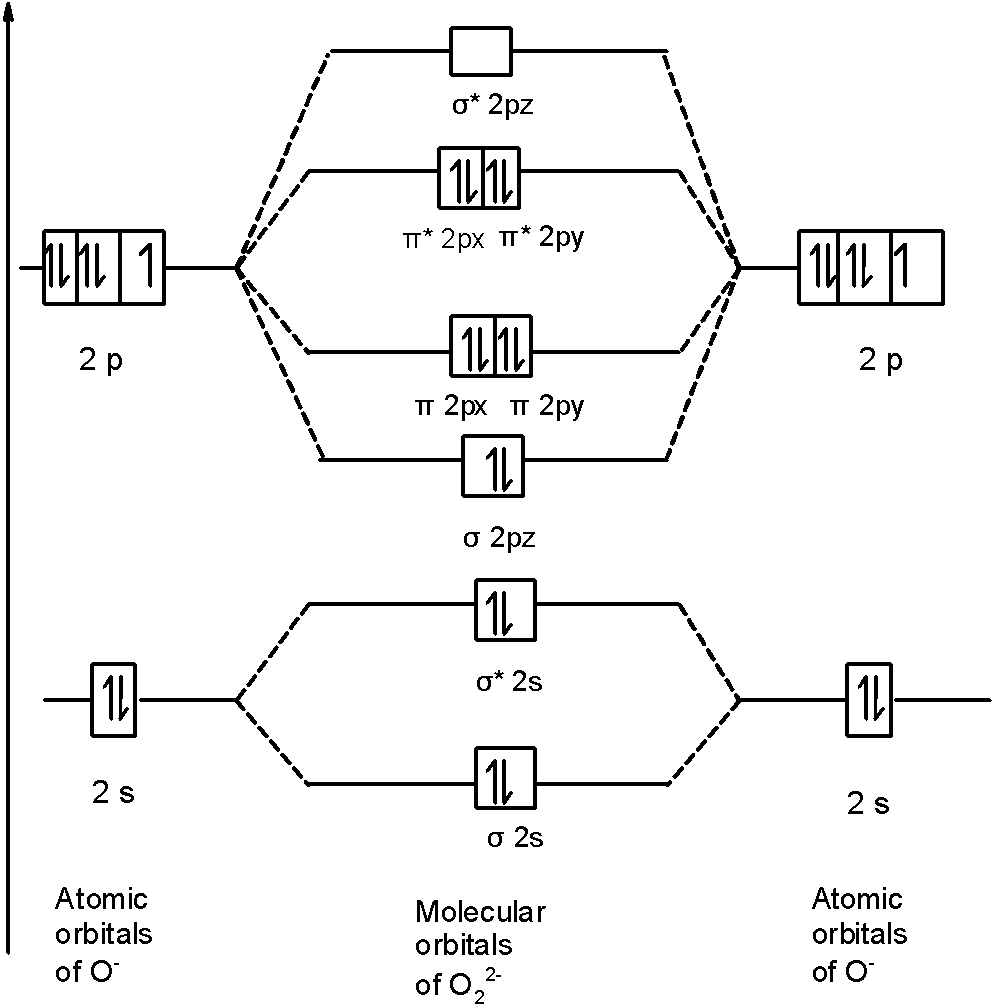
O2 2- mo diagram. Solved Sketch the molecular orbital (MO) diagram for O2^2 ... Sketch the molecular orbital (MO) diagram for O2^2 -. Is the molecule paramagnetic or diamagnetic? Calculate the bond order. Verify the bond order by drawing the Lewis Structure Is this ion more stable or less stable than elemental oxygen? WHY? Using the table of average bond energies below, calculate Delta H for the reaction below: H-C ... MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate ... Follow me on instagram- me on facebook page- ... Explain the formation of O2 molecule using molecular class ... $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - More stable among O2+ and O2- Chemical Bonding and ... O 2 + is more stable than O 2-. Reason: According to molecular orbital theory O 2 + has 15 electrons &it has one electron in antibonding orbital. molecular orbital diagram of O 2 + Electronic configuration of O 2 + In the case of O 2-17 electrons are present &3 electrons are present in antibonding orbitals. If number of electrons more in antibonding orbital the molecule become unstable.
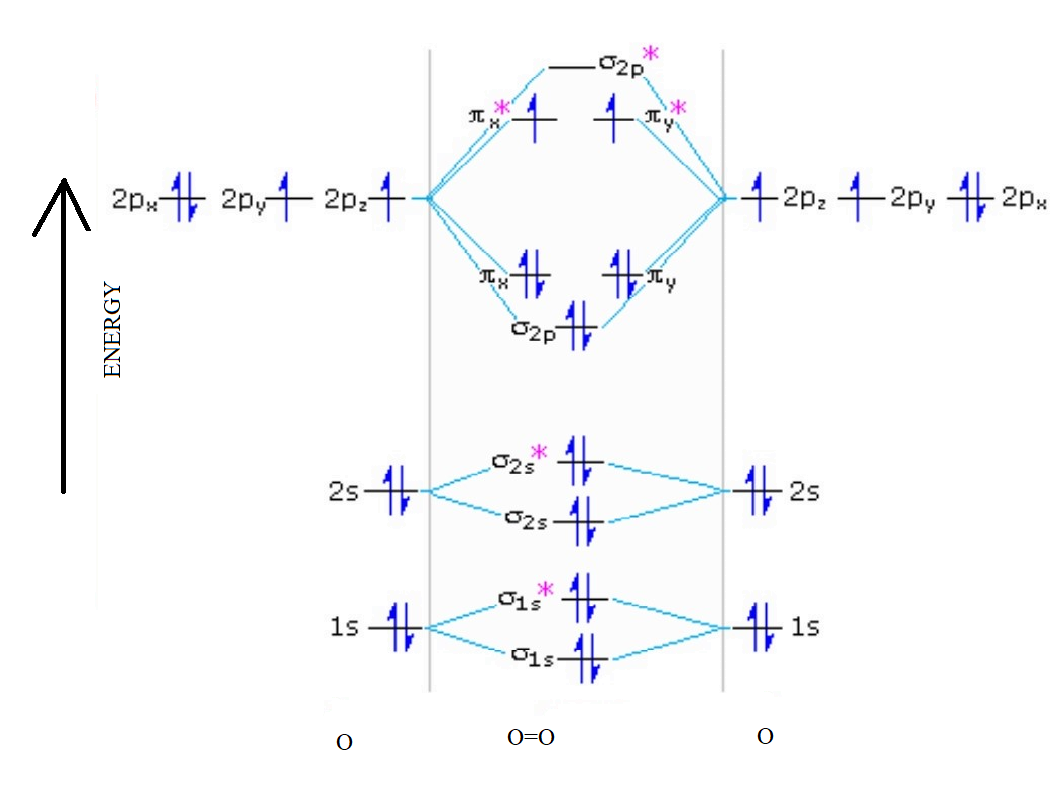
Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2. . , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. . −N a. . ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O. Solved Draw the MO diagram for Li2, Be2, B2, C2, N2 ... Draw the MO diagram for Li 2, Be 2 , B 2, C 2, N 2, N 2 +, O 2, O 2+2 , O 2-2, Ne 2, NO, NO -, CO, and CN - (list if it is paramagnetic or diamagnetic if possible) Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
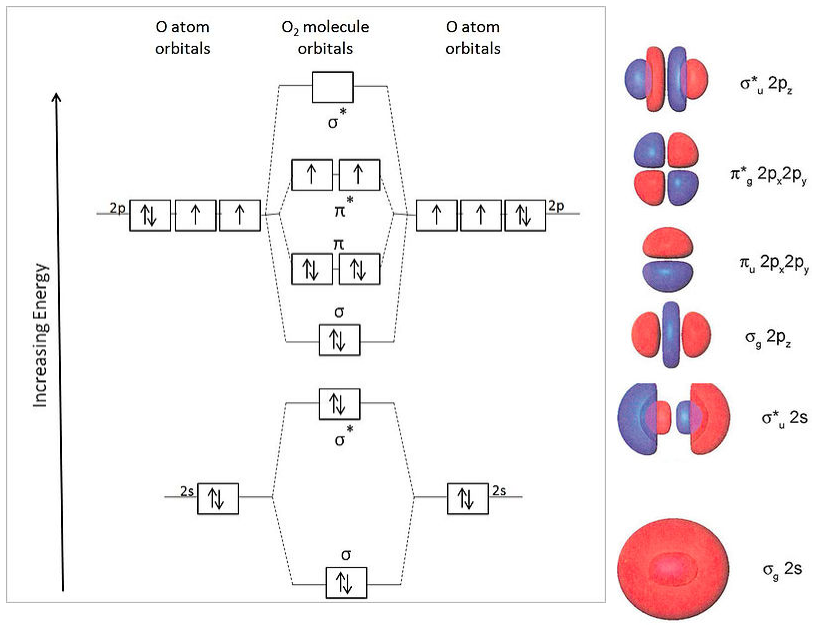
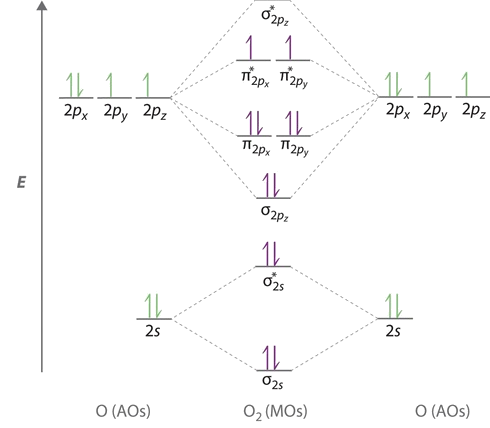
electronic configuration - Molecular orbital (MO) diagram ... I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. ... {O2+}$, the lower effective nuclear charge on nitrogen should make its s orbitals a little closer to the energies of the p orbitals than they would be in oxygen. Hence s-p mixing should occur ... MO Diagrams - GitHub Pages #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine PDF Energetic and chemical reactivity of atomic and molecular ... The molecular orbital diagram can be constructed from the molecular orbital theory (Figure 2). In molecular oxygen, there are 16 electrons which can be placed into the molecular orbitals to give the electronic configuration: (σ1s) 2(σ* 1s) 2(σ 2s) 2(σ* 2s) 2(σ 2p) 2 (σ* 2p) 2(π 2p) 4(π* 2p) 2. This electronic configuration shows that ...
In the molecular orbital diagram for O2^ + ion, the ... Verified by Toppr. Correct option is C) As it can be seen from the given structures that in the molecular orbital diagram for O 2+. . ion, the highest occupied orbital is π ∗ MO orbital.
Molecular Orbital Diagram Ne2 - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2.
MO Energy Level Diagram of O2, O2(+), O2(2+), O2 ... - YouTube In this Video we discussed about MO energy level diagram of O2 and thier ionic species. Bond order, Magnetic behavior of O2, O2(+), O2(2+), O2(-) and O2(2-) ...

Write the molecular orbital configuration of O(2)^(+) Calculate its bond order and predict its magnetic behaviour.
Molecular Orbital (MO) Diagram for O2(2+) - YouTube Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
Electronic valence molecular orbital configuration of "O ... You'll need the molecular orbital (MO) diagram of O2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get O2. Two 2s orbitals combine to give a σ2s bonding and σ* 2s antibonding MO.
What's the MOT diagram of O2 +2 ion? - Quora Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
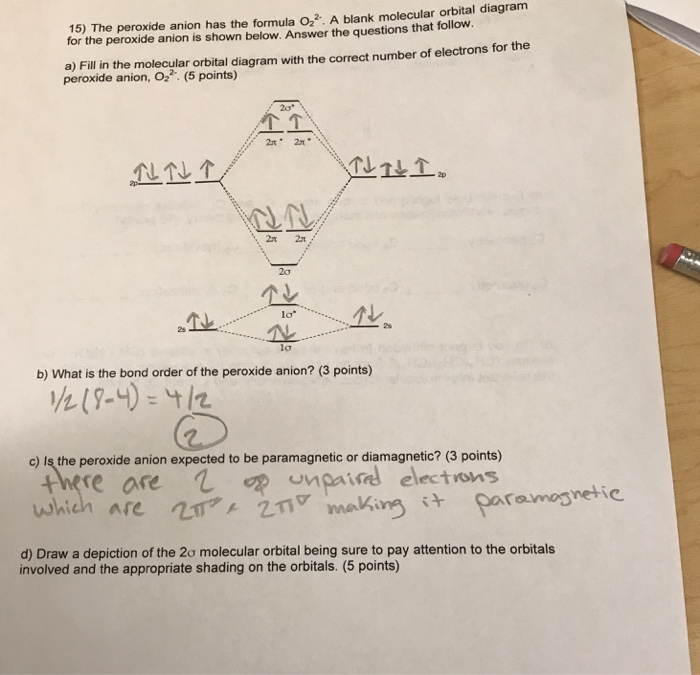
MO Diagram for O2(2-) - YouTube It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out:
The increasing order of the bond order of O2, O2-, O2+ and ... Bond order (B.O) 1/2 × [Number of an electron in antibonding molecular orbitals] - [Number of electrons in bonding molecular orbitals] The higher the order of the bond the greater the pull between the two atoms and the shorter the length of the bond. (1) B.O for O 2 = 1/2 × [10 - 6] B.O for O 2 = 2 (2) B.O for O 2 - = 1/2 × [10 - 7]
What's the MOT diagram of O2 +2 ion? - Quora In normal O2, there are 6 bonding electrons and 2 antibonding electrons, making the bond order 2. By removing the 2 highest electrons, which reside in ...3 answers · 9 votes: In O2 2+, there is 14 electrons. So, it’s MOT is comparable to N[code ]2[/code] & the MOT ...
N2+ Mo Diagram - schematron.org There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves.
N2+ Mo Diagram 2- = Molecular orbital for N2, N2+, O2, H2 and He2 by Thomas Wells - December 5, Brian Verfuerth 0. Ozone Lewis diagrams and by avatar Claire Bridget . The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves.






















0 Response to "38 o2 2- mo diagram"
Post a Comment