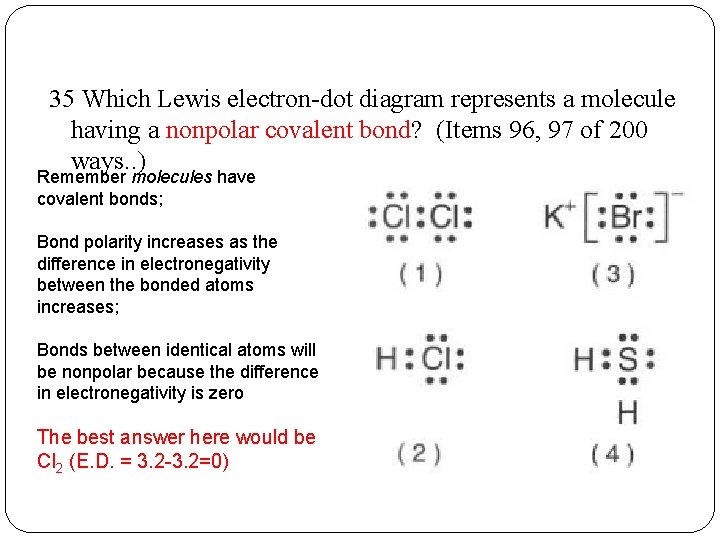
38 which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond
PDF Regents review Chemical bonding 2011-2012 - Mr. Palermo's ... 21.Which is the correct electron-dot formula for a hydrogen molecule at STP? A)F2 B)O2 C)Cl2 D)N2 22.Which molecule will have a double covalent bond? A)covalent bonding B)hydrogen bonding C)ionic bonding D)metallic bonding 23.Which type of bonding is found in all molecular substances? A)HI B)KI C)KCl D)LiCl 24.Which formula represents a ... A polar covalent bond would form in which one of the ... 3.3.2022 · Maybe 9. Polar covalent bonds are characterized by atoms with uneven or unequal numbers or the sharing of electrons between the two electrons. Nonpolar covalent bonds form when there is a DEN between 0 and 0. P - C. 3: The bond is nonpolar covalent. In a polar bond there are partial charges on the atoms sharing electrons. 12.
Which lewis electron-dot diagram represents a molecule having ... The diagram below represents a portion of a dna molecule Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell A single covalent bond contains ________ of electrons.
Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond
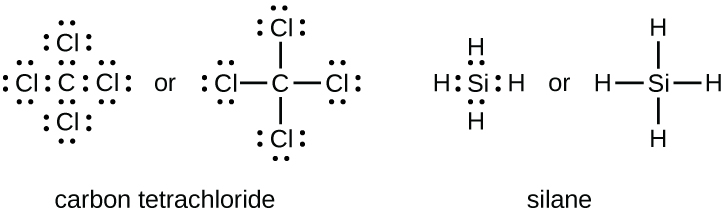
PDF St. Francis Preparatory School Explain, in terms of structure and/or distribution of charge, why the C02 electron-dot diagram shown is a nonpolar molecule. Explain, in terms of electronegativity, why the CFO bond in C02 is more polar than the bond in F2 in the diagrams shown. PDF Chapter 16 Covalent Bonding - MRS. MORALES PEP SITE Bond dissociation energy is defined as the energy needed to break one covalent bond. 45. Assume the total bond energy in a molecule is the sum of the individual bond energies. Calculate the total bond energy in a mole of ethyne (C 2 H 2). Hint: Write the electron dot structure to determine the kinds of bonds. Then refer to Table 16.3. 46. ClF5 Lewis Structure, Molecular Geometry, Hybridization ... 4.3.2022 · Lewis Structure is also known as an electron-dot structure since it uses dot notations to represent the valence shell electrons in the skeletal diagram. Here, as we can see, we have put all the 42 electrons surrounding the six atoms in ClF5. Since Chlorine is the central atom here, it will form bonds with all the five Fluorine atoms.
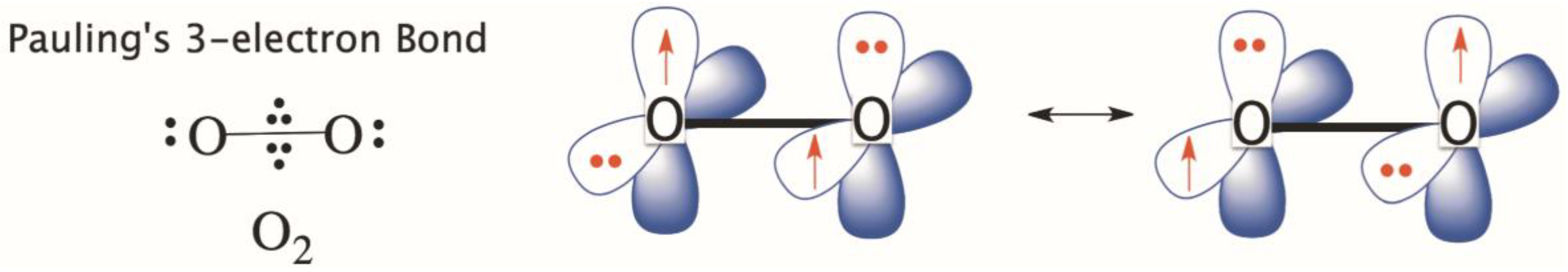
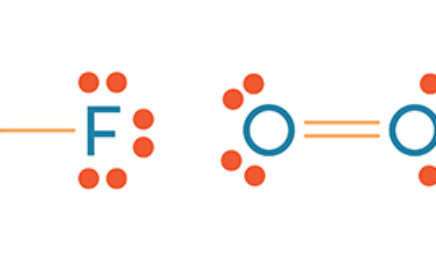
Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond. Lewis Dot Diagram For O2 - schematron.org Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. So as you may of remember from Chemistry ... Covalent Bond: Types of Bonds, Examples, Formation - Embibe A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom, the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Which lewis electron dot diagram represents a molecule ... Correct answer to the question Which lewis electron dot diagram represents a molecule having a nonpolar covalent bond. - hmwhelper.com. Subjects. English; ... Which lewis electron dot diagram represents a molecule having a nonpolar covalent bond.... Questions in other subjects: Chemistry, 30.08.2019 04:10 ... Which electron dot diagram represents a molecule that has a ... Jan 16, 2018 · heart. 7. 7. AthenaeumSagas. AthenaeumSagas. Interlocking circles are seen in the electron dot diagram that represents a molecule that has a polar covalent bond.
CH4 lewis structure, Molecular geometry, Polar or nonpolar ... CH4 is a nonpolar molecule due to its symmetrical geometry that causes uniform charge distribution all over the atom leads to a zero net dipole moment and makes this molecule non-polar in nature. The molecular geometry or shape for CH4 is the tetrahedral with bond angle ∠H−C−H =109.5°. Regents Chemistry Exam Explanations January 2012 35 Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond? 1: link . 2 of the same element bonded= nonpolar bond: 36 Which quantity is equal to 50 kilojoules? (I ) 0.05J (3) 5 x 10 3 J (2) 500J (4) 5 x 10 4 J: 4 : first replace kilo with x 10 3 (table C prefixes) 50 x 10 3 which is 5 x 10 4 Covalent Bonding: Electron Dot Diagrams - Texas Gateway In covalent bonding, nonmetallic elements share electrons so that both elements can have a full valence shell. Covalent bonds can be represented with electron dot formulas. These are often referred to as Lewis structures and are a little different than the electron dot formulas used to represent ionic bonds. Which formula represents a molecule having a nonpolar ... Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond; Which factor distinguishes a metallic bond from an ionic bond or a covalent bond; Which formula represents a molecule with the most polar bond; Which statement explains why a molecule of ch4 is nonpolar
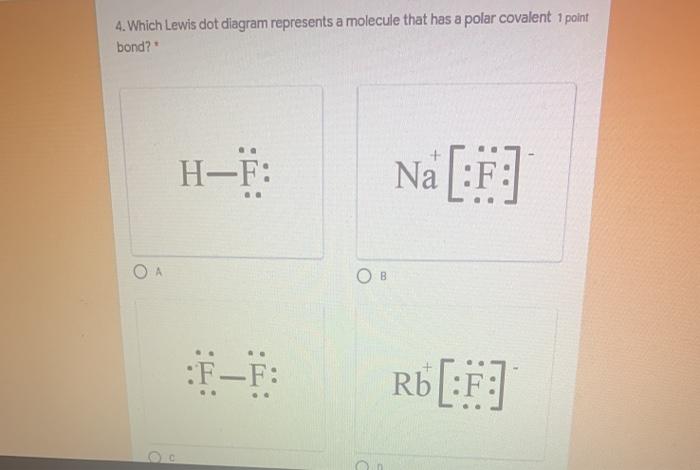
PDF Unit 4 Bonding Exam Name 14) The bond between hydrogen and oxygen in a water molecule is classified as a) covalent and nonpolar c) ionic and polar b) ionic and nonpolar d) covalent and polar 15) Which is a nonpolar molecule containing a nonpolar covalent bond? a) I 2 b) CO 2 c) NH 3 d) H 2O 16) Which diagram best represents a polar covalent molecule? PDF 3.4 Covalent Bonds and Lewis Structures two atoms constitutes a covalent bond. The Lewis Model of Chemical Bonding. Covalent Bonding in H2 ... •Both have the molecular formula CH3NO2 but the atoms are connected in a different order... H CC OO N O H H..:: ... assigned to specific atoms-a single Lewis structure is insufficient to show electron delocalization. ai-team.it N2 lone pairs 2 3 4 28 Which formula represents a molecule having a ... In the space draw a Lewis electron-dot diagram for the reactant containing nitrogen in the equation. 39. Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule.. on
PDF Bonding TEACHER ANSWER KEY Mrs. Pugliese 3 - Quia 3 In a Lewis electron-dot diagram, the valence (outer-level) electrons of the relevant atoms are represented by one or more pairs of dots. 3 21. Which formula represents a nonpolar molecule? 1. H 2 S 3. CH 4 2. HCl 4. NH 3 3 Nonpolar molecules must have symmetrical electron distribution. Of the choices given, only
Electron Configurations Flashcards - Quizlet which Lewis electron dot diagram represents a molecule having a non-polar covalent bond. symmetrical and non polar. which phrase describes the distribution of charge in the polarity of a CH4 molecule? Cl:Cl. ... Which one of the following formulas represents a molecule having a nonpolar covalent bond.
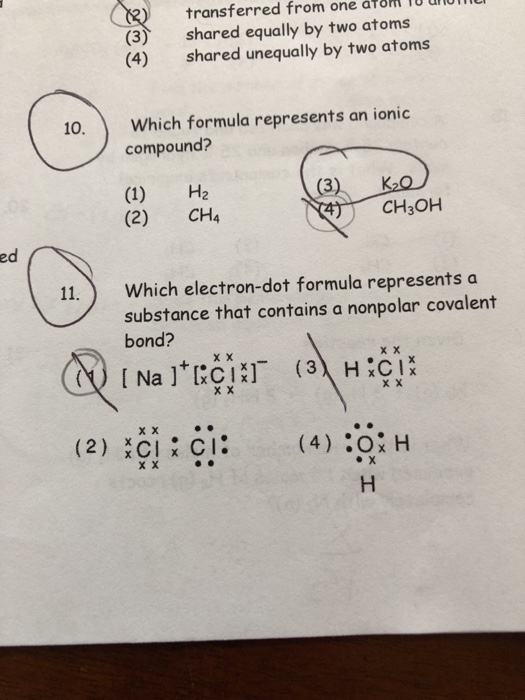
PDF Lecture B1 Lewis Dot Structures and Covalent Bonding formalism for representing covalent bonding in molecules. a "single bond", consisting of a pair of electrons. Lewis dot structures provide a simple, but extremely powerful, formalism for representing covalent bonding in molecules. a nonbonding pair of electrons: a"lone pair" Lewis dot structures provide a simple, but extremely powerful,
Which Lewis electron-dot diagram represents a molecule ... Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond? Answers: 2. Show answers.
PDF Name CHEMICAL BONDING REVIEW Date Ms. Zavurov Per 39.Which electron-dot diagram represents a molecule that has a polar covalent bond? A)ionic B)metallic C)polar covalent D)nonpolar covalent 40.Which type of bond exists between an atom of carbon and an atom of fluorine? A)ionic B)electrovalent C)polar covalent D)nonpolar covalent 41.Which type of bond is formed between the
How do you determine if a molecule has a polar covalent ... When you draw the lewis dot structure can you draw a circle around both atoms that represent a stable inert gas structure. In a covalent bond the electrons are shared between both atoms, in a roughly equal manner. If the electron structures for both atoms are stable after sharing they will share the electrons covalently. CO_2 as an example .. .. : O :: C :: O : If you draw a circle around ...
CO2 lewis structure, molecular geometry, bond angle, polar ... CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule.
Covalent Bond | Lewis Bonding Theory | Dot Model ... This in turn is shared between the two hydrogen atoms to form a covalent bond. * Thus in H 2 molecule, each hydrogen atom gets its nearest inert gas: Helium's configuration, 1s 2. * The covalency of hydrogen is 1. * The Lewis dot structure for H 2 molecule is shown below. Note that each hydrogen gets two electrons after forming the bond.
20 Which Lewis electron dot diagram represents a molecule ... Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond? 1) 24 2) 26 3) 28 4) 56 21. How many electrons are in an Fe 2+ ion Electronegativity decreases and atomic radius increases. The nuclear charge of each successive atom decreases, and the atomic radius decreases.
PDF Test 8: Review Questions Name: Thursday, February 14, 2008 Which formula represents a nonpolar molecule containing polar covalent bonds? 1. H O 3. NH2 3 2. CCl 4. H4 2 29. When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of chlorine 1. loses an electron 3. becomes an ion with a smaller radius than the atom of chlorine
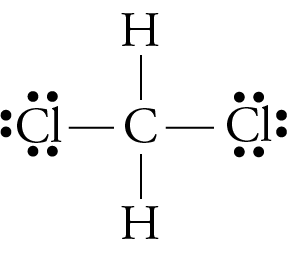
PDF Bond and Molecular Polarity - Forest Hills High School 4.Which formula represents a molecule having a nonpolar covalent bond? A)H2O B)CCl4 C)NH3 D)H2 5.Which formula represents a nonpolar molecule containing polar covalent bonds? A)CH4 B)HCl C)H2O D)NH3 6.Which formula represents a nonpolar molecule? A)Electrons are shared between the carbon atoms
Name: Bonding Review 28.Which formula represents a molecule having a nonpolar covalent bond? 1)C–N 2)H–H 3)S–Cl 4)Si–O 29.The chemical bond between which two atoms is most polar? 1)CH4 2)CaH2 3)KH 4)NH3 30.Which compound has hydrogen bonding between its molecules? 1)H2O 2)CCl4 3)NH3 4)H2 31.Which formula represents a nonpolar molecule containing polar ...
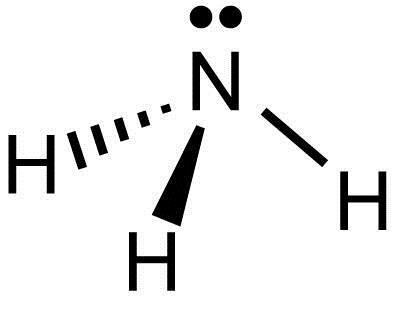
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule's atoms and the lone pairs of electrons that may occur in the molecule. What is the Lewis structure of ammonia? Ammonia has the NH3 equation. It is extremely water-soluble because it is a polar material.
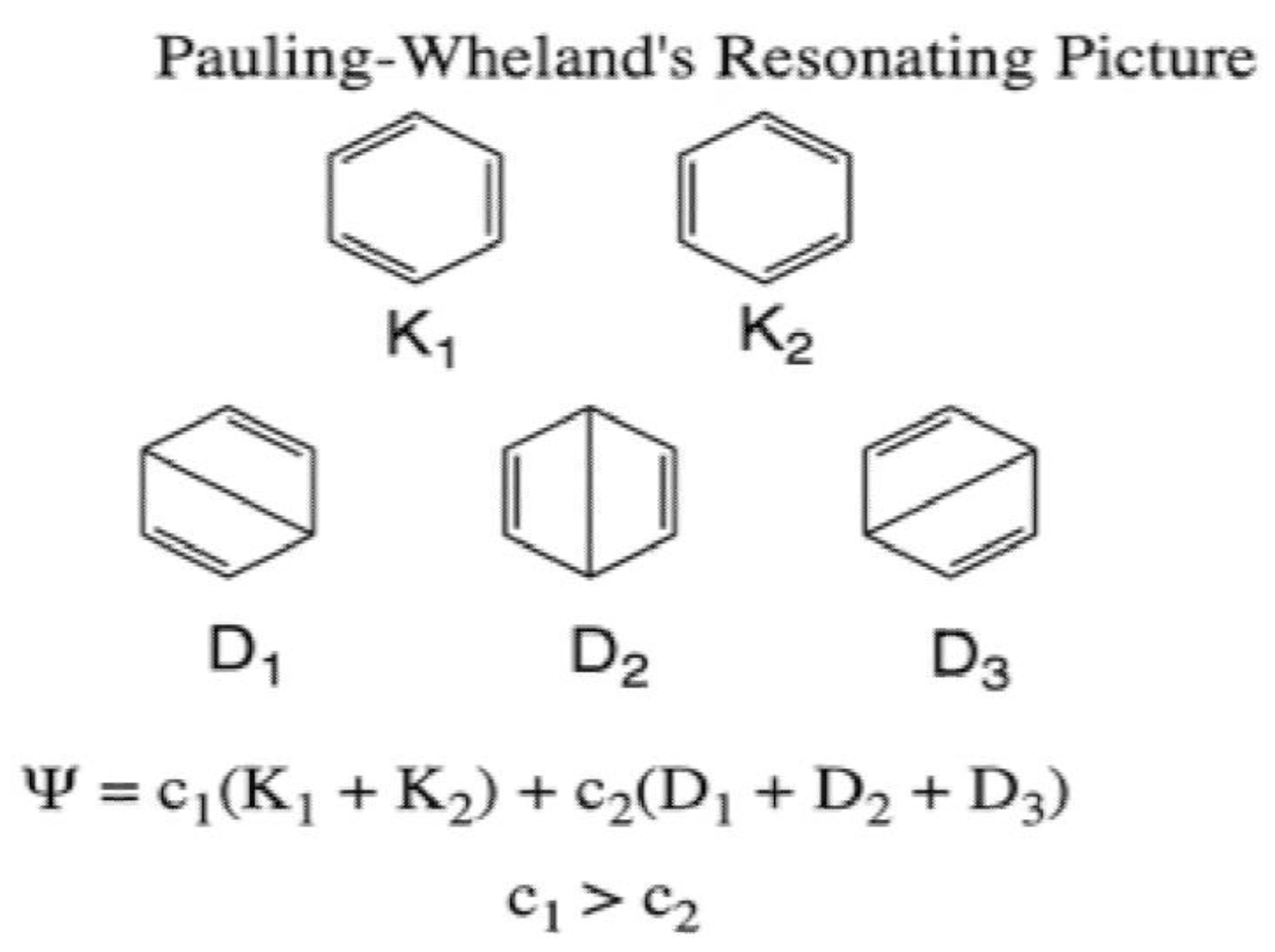
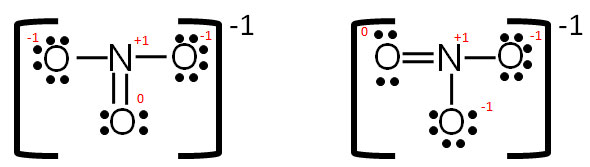
Lewis Structures and the Shapes of Molecules In this example, we can draw two Lewis structures that are energetically equivalent to each other — that is, they have the same types of bonds, and the same types of formal charges on all of the structures.Both structures (2 and 3) must be used to represent the molecule's structure.The actual molecule is an average of structures 2 and 3, which are called resonance structures.
Manhasset Union Free School District / Homepage 2. Which Lewis electron-dot diagram represents the bonding in potassium iodide? 3. Which equation shows conservation of mass and energy for a reaction at 101.3 kPa and 298 K? + 483.6 4. + 285.8 kJ 4. Given the formulas of two substances: These diagrams represent substances that have 1. the same molecular structure and etthe same physical properties
Topic 6 Bonding Flashcards - Quizlet Which electron dot diagram represents a molecule that has a polar covalent bond? HCl When a sodium atom reacts with a chlorine atom to form a compound, the electron configuration of the ions forming the compound are the same as those in which noble gases
ClF5 Lewis Structure, Molecular Geometry, Hybridization ... 4.3.2022 · Lewis Structure is also known as an electron-dot structure since it uses dot notations to represent the valence shell electrons in the skeletal diagram. Here, as we can see, we have put all the 42 electrons surrounding the six atoms in ClF5. Since Chlorine is the central atom here, it will form bonds with all the five Fluorine atoms.
PDF Chapter 16 Covalent Bonding - MRS. MORALES PEP SITE Bond dissociation energy is defined as the energy needed to break one covalent bond. 45. Assume the total bond energy in a molecule is the sum of the individual bond energies. Calculate the total bond energy in a mole of ethyne (C 2 H 2). Hint: Write the electron dot structure to determine the kinds of bonds. Then refer to Table 16.3. 46.
PDF St. Francis Preparatory School Explain, in terms of structure and/or distribution of charge, why the C02 electron-dot diagram shown is a nonpolar molecule. Explain, in terms of electronegativity, why the CFO bond in C02 is more polar than the bond in F2 in the diagrams shown.



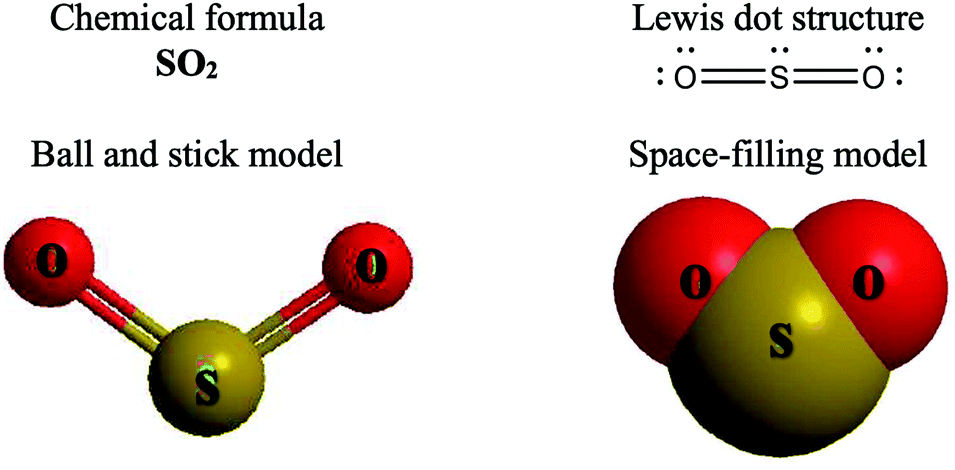

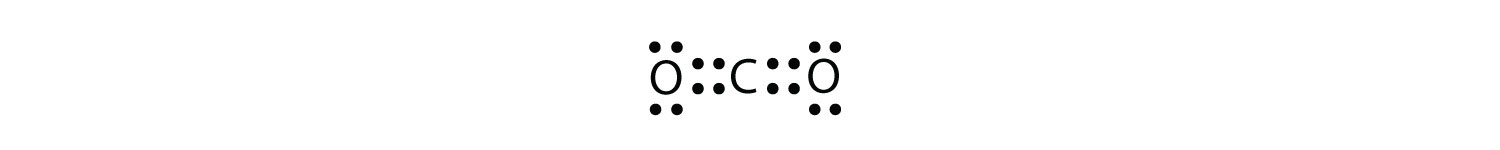









0 Response to "38 which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond"
Post a Comment