40 boron lewis dot diagram
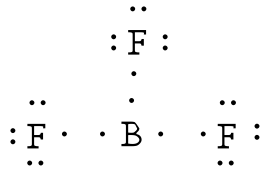
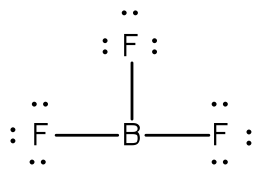
Lewis Dot Structures | ChemTalk Feb 21, 2021 — Learn and master lewis dot structures with this easily digestible tutorial ... Since boron has 3 valence electrons, there will be 3 dots all ... Lewis Structure for BF3 (Boron Trifluoride) - UMD Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the structure. Boron is an exception and only needs 6 valence electrons in its outer shell. If we check the formal charges for the BF 3 Lewis structure we will find that they are zero even though B only had six valence electrons.
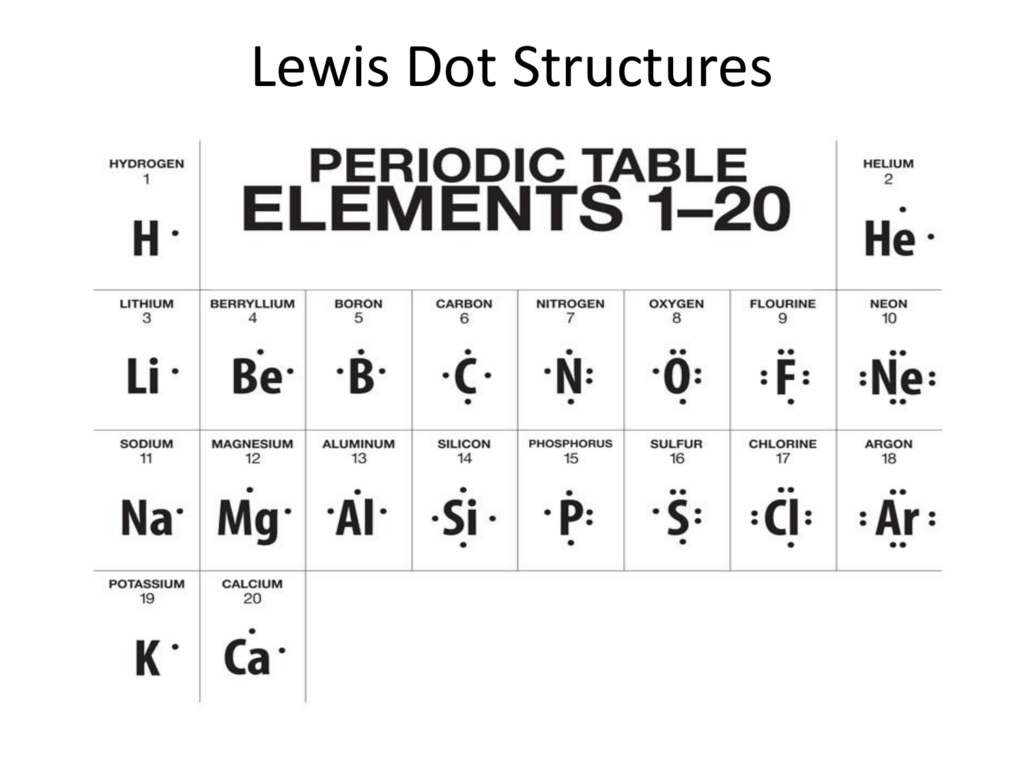
9.1 Lewis Electron Dot Diagrams A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Boron lewis dot diagram
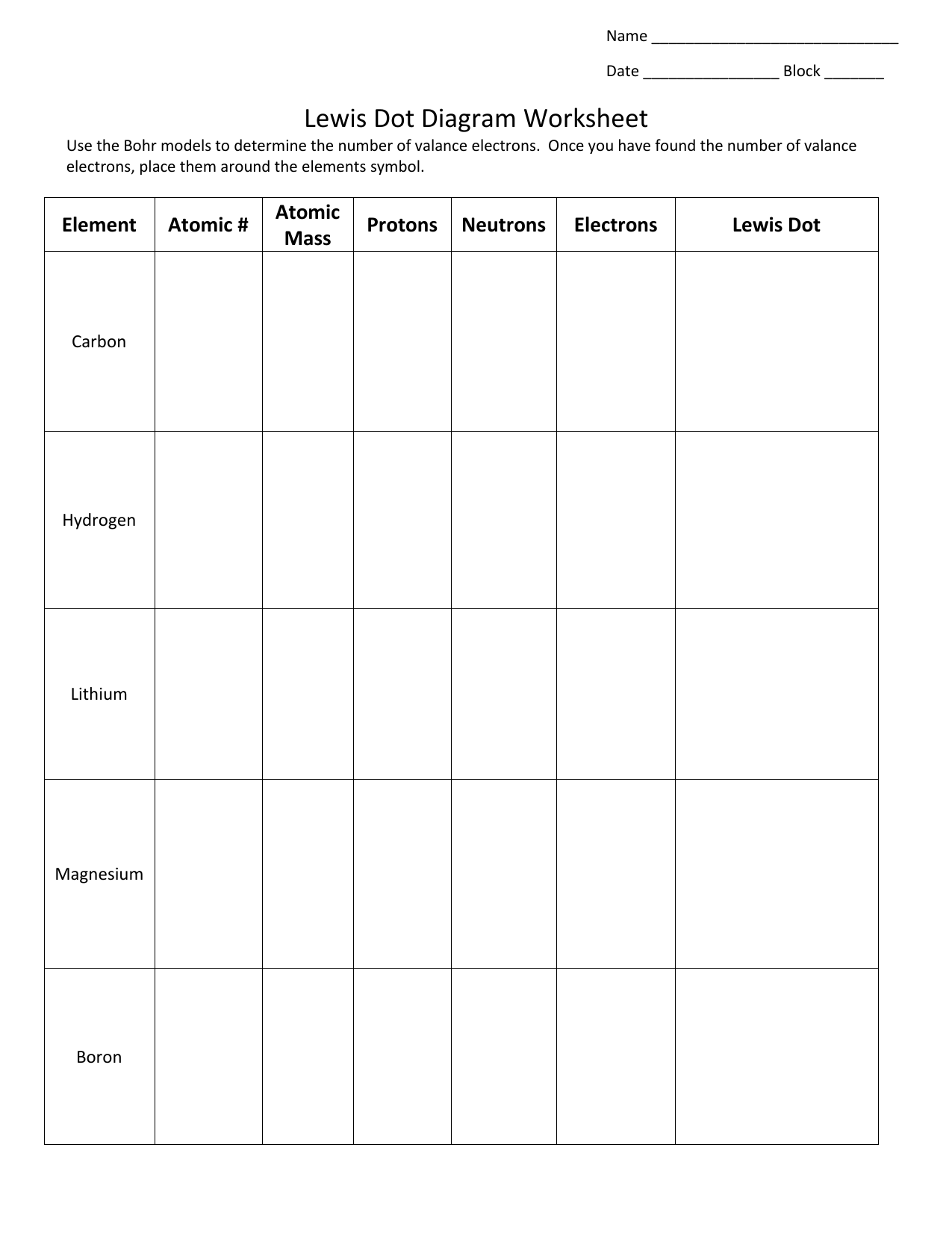
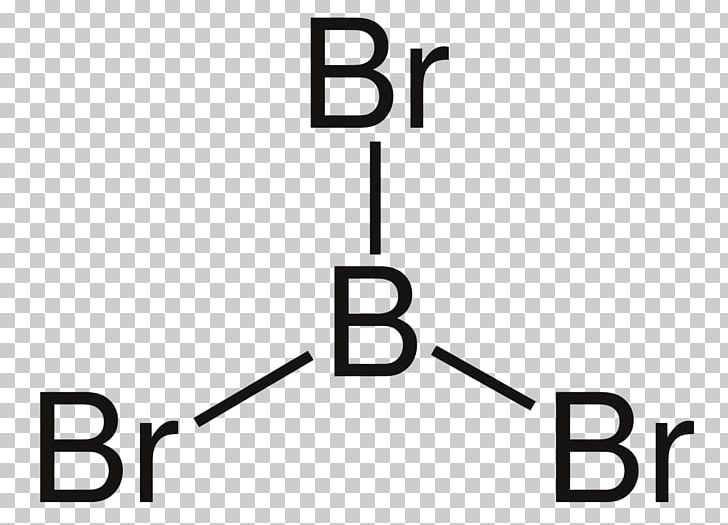
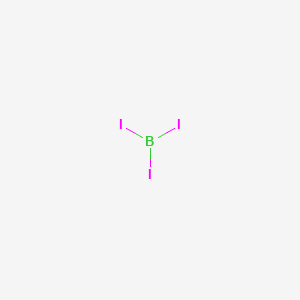
Lewis Electron- Dot Structure of boron fluoride BF ... Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 A simple and general method for writing Dot Structures - Lewis Structures is given in a previous article entitled "Lewis Structures and the Octet Rule". Relevant worked examples were given in the following articles: ... Lewis Dot Diagram For Boron Sep 20, 2018 · Boron is the central atom surrounded by three iodines with single bonds, and remember boron only need six electrons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Hope this helps!. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions ... Boron tribromide (BBr3) lewis dot structure, molecular ... Boron tribromide (BBr3) lewis dot structure, molecular geometry, polar or nonpolar, hybridization Home > Chemistry Article > BBr3 lewis structure and its molecular geometry Boron tribromide composed of boron and bromine appears as colorless to amber liquid, has a sharp and irritating odor with chemical formula BBr3.
Boron lewis dot diagram. How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ... Lewis Structure For Boron Oxide - Novocom.top Lewis Structure For Boron Oxide, BCl3 Lewis Structure, Molecular Geometry, Hybridization, Incomplete Octet in Boron Trifluoride (BF3) Molecule QS, Lewis Dot Structure of BH3 (Boron Hydride) YouTube, Lewis Acids and Bases Chemistry 2e How to draw BBr3 Lewis Structure? - Science Education and ... It is represented by dots in the BBr3 Lewis diagram. The BBr3 molecule's core boron atom can be represented as follows: Total outermost valence shell electron of boron atom in BBr3= 3 Total outermost valence shell electron of the bromine atom in BBr3= 7 The BC3 molecule has one central boron and three bromine atoms. BF3 Lewis Structure, Molecular Geometry, and Hybridization 24/02/2022 · Finding out Lewis Structure of BF3. The periodic table helps you to study various elements that include atomic number, valency, etc. To learn about any Lewis dot structure of boron trifluoride BF3, you need to compute mainly four important things. The total number of valence electrons. Required number of electrons to complete octet.
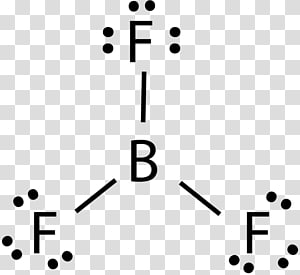
5.3: Lewis Diagrams - Chemistry LibreTexts 15/07/2021 · Draw Lewis diagrams for an atom of each of the following elements: Li, N, F, Na. Solution. We find from the periodic table inside the front cover that Li has an atomic number of 3. It thus contains three electrons, one more than the noble gas He. This means that the outermost, or valence, shell contains only one electron, and the Lewis diagram is BF3 Lewis structure, Molecular geometry, Hybridization ... Steps for drawing Lewis dot structure of BF3 Count the total number of valence electrons present on each atom of BF3 The total number of valence electrons of the BF3 molecule is 24. Boron lies on group 13 in the periodic table and contains a valency of 3. Fluorine lies on group 17 in the periodic table and contains a valency of 7. 7.3 Lewis Symbols and Structures – Chemistry A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: ... BeH 2, and boron trifluoride, BF 3, the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence … Lewis Structure of Borane (BH3) - chemistryscl.com Lewis Structure of Borane (BH. 3. ) Borane (BH 3) is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane (BH3). There are only three bonds around boron atom and no lone pairs on boron atom.
Lewis Dot Structure For Boron Trifluoride - Novocom.top lewis boron dot diagram octet rule trifluoride electrons structures bf3 electron diagrams sundin bonds obey covalent always dimensional bf three . bf3 bond lewis boron structure angle bonding trifluoride covalent electrons electron angles bonds vias pairs sharing genchem figure extensions fluorine . Lewis Structure of Boron Trifluoride (BF3) BF 3 lewis structure. According to the lewis structure of BF 3, there are only six electrons around boron atom.Therefore, octal of boron atom is not completed. Therefore, borane BF 3 is considered as a lewis acid.. Steps of drawing lewis structure of BF 3. There are general guidelines to draw a lewis structure step by step and they are mentioned below. Lewis Dot Diagram For Boron Feb 16, 2019 · Lewis Dot Diagram For Boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Because experiments suggest that there is some degree of overlap. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical dot diagram ... Draw and explain the Lewis dot structure of boron. | Study.com Boron has an elemental symbol as "B" and it has three electrons in the valence shell i.e. two electrons in 2s and 1 electron in 2p.
Aligned Stacking of Nanopatterned 2D Materials for High ... For example, hexagonal boron nitride (hBN) is not only an excellent ... in the support frame (e.g., TEM grid), is patterned by focused electron irradiation (modified area indicated as red dot). (b,c) The membrane is moved (under SEM observation) onto a second support with a smaller hole. A slight curvature of the second support frame makes it possible to bring the two surfaces into …
Lewis Dot Structure for Boron Oxide B2O3 Boron metal has 3 ... Lewis Dot Structure for Boron Oxide (B2O3) Boron (metal) has 3 valence electrons Oxygen (nonmetal) has 6 valence electrons, needs 2 to be balanced B3↘ ↙O2 B2 O3 Lewis Dot Structure for Sodium Fluoride Na +1 Cl-1 Na has 1 valence electron Fl needs 1 to be balanced Na Cl Covalent Bonds Covalent Bonds are attractions of 2 nonmetals They have low melting/boiling points and aren't a good ...
Potassium Bohr Model - How to draw Bohr diagram for ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Potassium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electron around the Potassium atom as a dot.
How to draw BCl3 Lewis Structure? - Science Education and ... To sketch the BCl3 Lewis structure by following these instructions: Step-1: BCl3 Lewis dot Structure by counting valence electrons on the boron atom. Step-2: Lewis Structure of BCl3 for counting valence electrons around the terminal chlorine atom. Step-3: Lewis dot Structure for BCl3 generated from step-1 and step-2.
BF3 Lewis Structure (2022 UPDATED) Practical Guide In BF3, Boron is the least electronegative atom and will be used as the central atom. The Lewis Dot Structure will show you one Boron atom with three electrons in its last shell and three Fluorine atoms with seven electrons in its last shell. The computation will end with 24 total valence electrons, forming three B F bonds.
Lewis Dot Structure for Boron Atom (B) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for B (Boron). I show you where Boron is on the periodic table and how to determine how ma...
Lewis dot structure of boron? - Answers This is an ionic compound. Sodium is positively charged and is paired with the negatively charged BH4 molecule, which, in Lewis dot structure form, comprises a boron atom connected to four H atoms.
Low-dimensional non-metal catalysts: principles for ... 16/11/2021 · Activation of p-block elements to replace the rare and precious transition metals for renewable energy applications is highly desirable. In this …
BCl3 Lewis Structure, Molecular Geometry, and ... BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.
boron monoxide lewis structure - timbasilering.com boron monoxide lewis structure. by | Mar 1, 2022 | lamps plus bedroom lamps | eobard thawne first appearance | Mar 1, 2022 | lamps plus bedroom lamps | eobard thawne first appearance
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
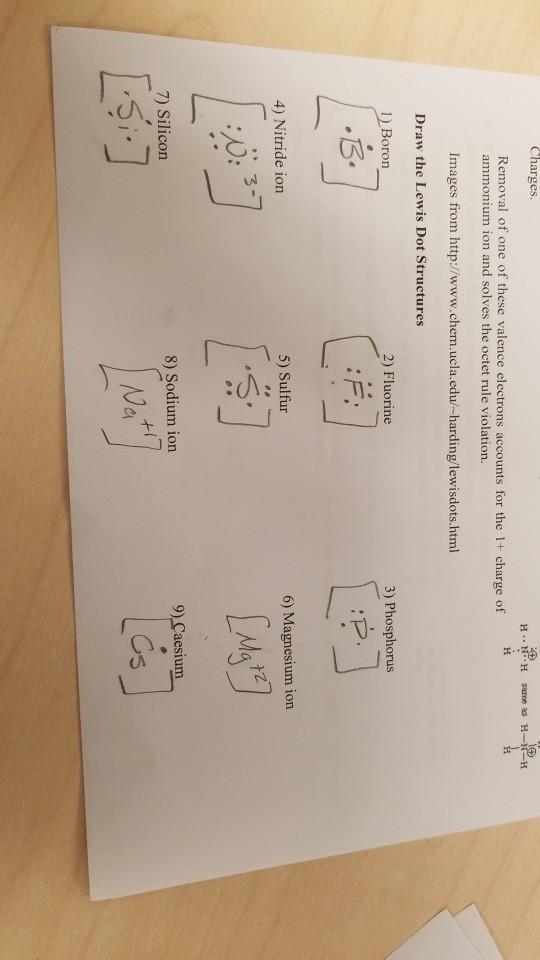
PDF Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon Hydrogen Lithium Magnesium Boron
Why is Boron Trifluoride written in two ways in the lewis ... Its formula is = [no. of valence electrons on atom] - [non-bonded electrons + number of bonds]. or a simple one {if no lone pair are there} = 1/2 no. of bonds.)on each atom. Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything. (in fig. 1). We must examine the formal charges of this structure.
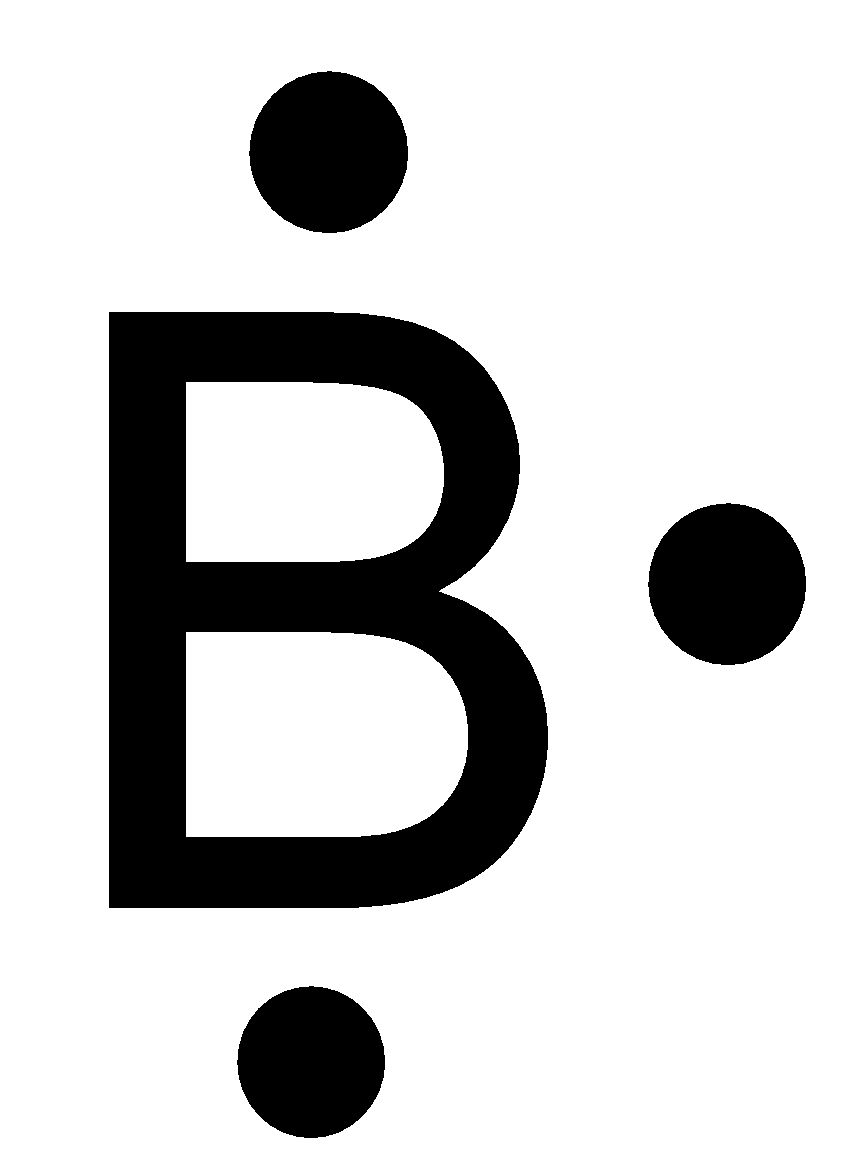
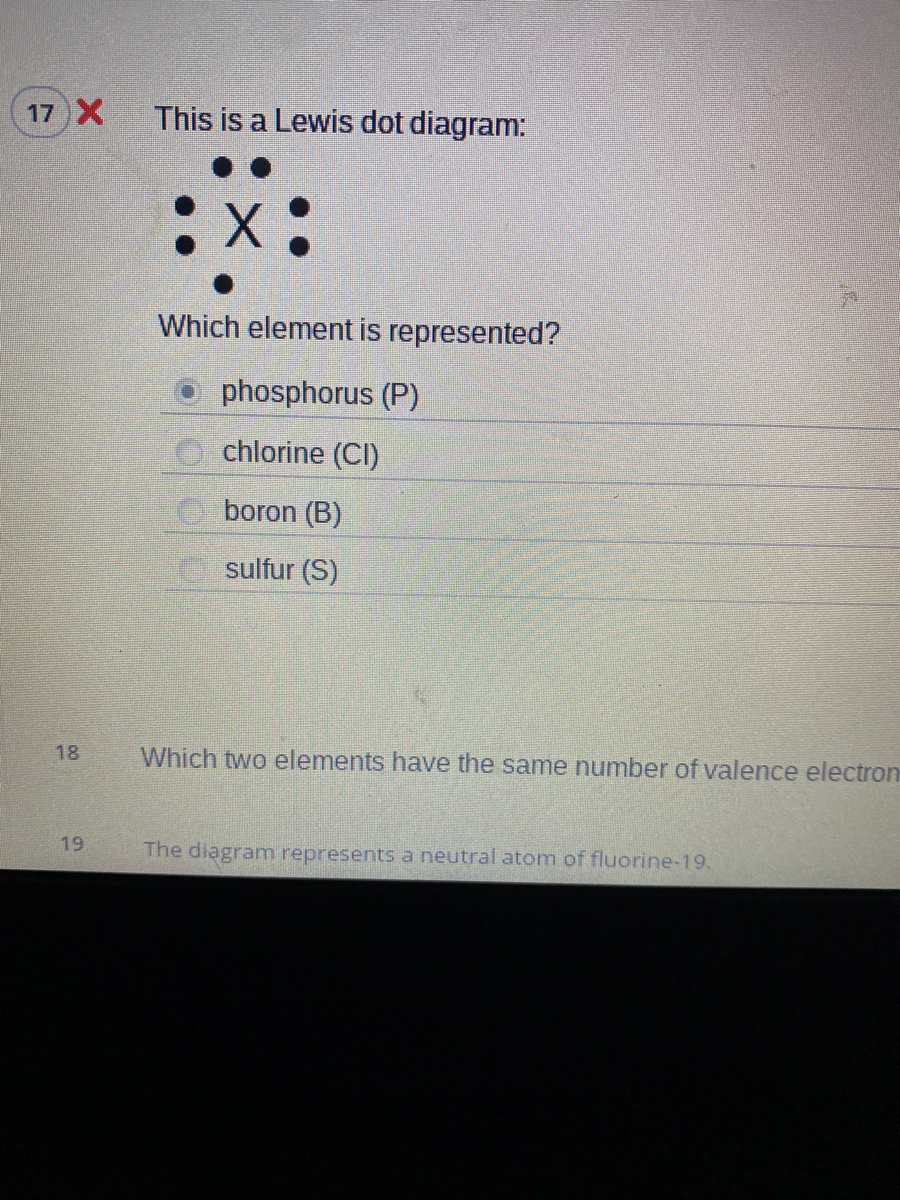
Which lewis electron dot diagram represents a boron atom ... Answer : The Lewis-dot structure of boron atom is shown below. Explanation : Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valance electrons are shown by 'dot'.
What is the Lewis dot structure for boron iodide? - Answers Boron is the central atom surrounded by three iodines with single bonds, and remember boron only need six electrons. And this lewis structure has no resonance and makes a polar molecule with a ...
Boron triiodide (BI3) lewis dot structure, molecular ... Hence, put the boron atom at the central position of the lewis diagram and all three iodine atoms outside to it. 3. Connect outer atoms to central atom with a single bond In this step, join all outer atoms to the central atom with the help of a single bond. In, BI3 molecule, iodine is the outer atom, and boron is the central atom.
How do you find the electron dot diagram for boron? - Quora The Lewis dot structure of boron nitride is determined by counting the number of valence electrons for both boron and nitrogen. Boron has three valence ...
Boron Lewis Dot Structure | Borates Today Dec 15, 2021 · Boron Lewis Dot Structure. Lewis dot structure is the structure of an element or molecule, and total valence electrons are as dots to represent the bond pairs and lone pairs. Boron has an elemental symbol as “B,” and the electronic configuration counts as 2,3, its atomic number 5. Hence, it has three electrons in the valence shell, i.e ...
Boron Bohr Diagram - schematron.org A Bohr diagram can be used to visually show the Bohr model of a particular atom. The Bohr diagram for boron shows a central nucleus containing five protons. Bohr Models and. Lewis Dot Structures. Page 2. Bohring. Page 3. Bohr/Lewis Dot Models. Used to predict Draw the Bohr Model for Boron.
How to find the electron dot diagram for boron - Quora Answer (1 of 2): A chemistry book perhaps. Boron has 5 electrons. 3 are in the valence shell. So Boron can use those electrons to bond with three other atoms. . B . . Here is a table showing the first 20 atoms (by atomic number).
Boron tribromide (BBr3) lewis dot structure, molecular ... Boron tribromide (BBr3) lewis dot structure, molecular geometry, polar or nonpolar, hybridization Home > Chemistry Article > BBr3 lewis structure and its molecular geometry Boron tribromide composed of boron and bromine appears as colorless to amber liquid, has a sharp and irritating odor with chemical formula BBr3.
Lewis Dot Diagram For Boron Sep 20, 2018 · Boron is the central atom surrounded by three iodines with single bonds, and remember boron only need six electrons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Hope this helps!. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions ...
Lewis Electron- Dot Structure of boron fluoride BF ... Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 A simple and general method for writing Dot Structures - Lewis Structures is given in a previous article entitled "Lewis Structures and the Octet Rule". Relevant worked examples were given in the following articles: ...












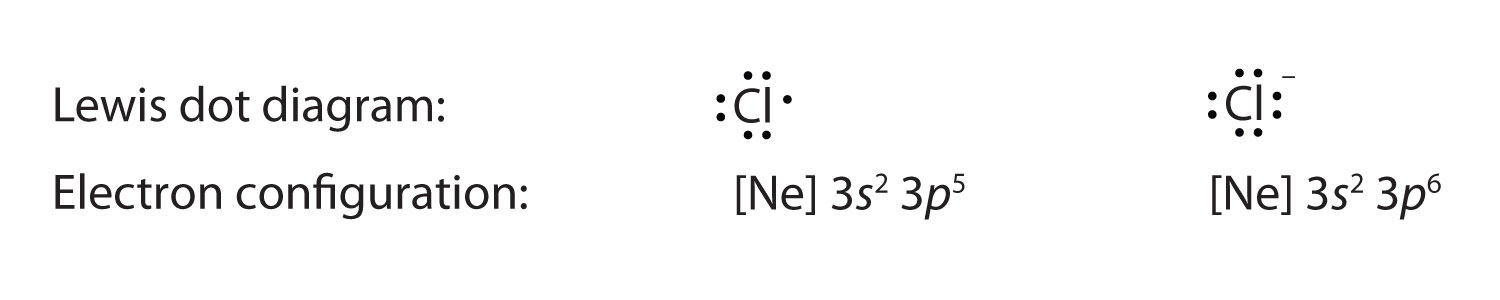


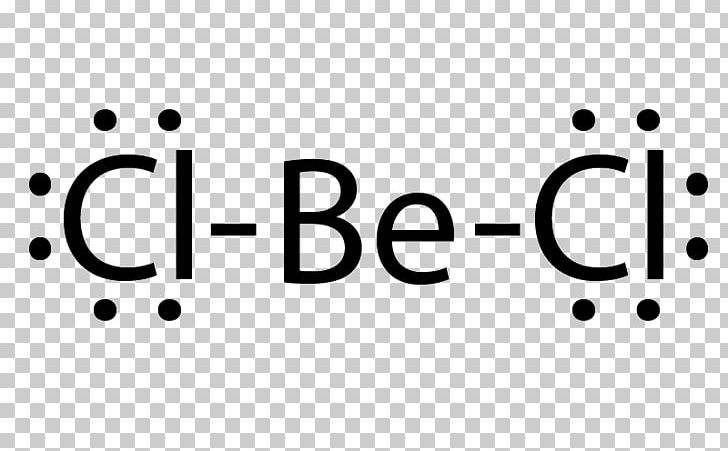
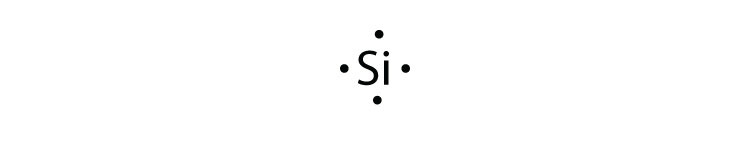



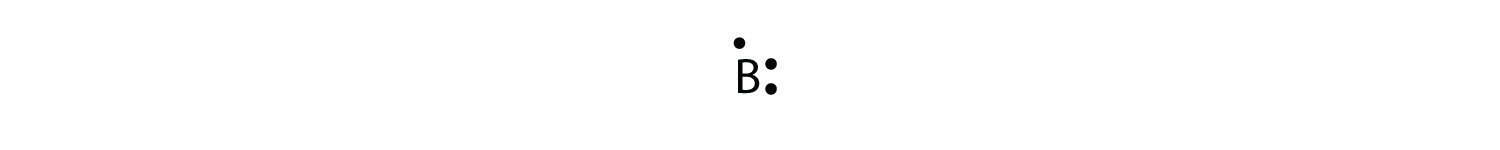


0 Response to "40 boron lewis dot diagram"
Post a Comment