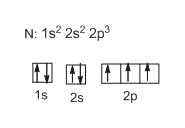
40 orbital filling diagram for sulfur
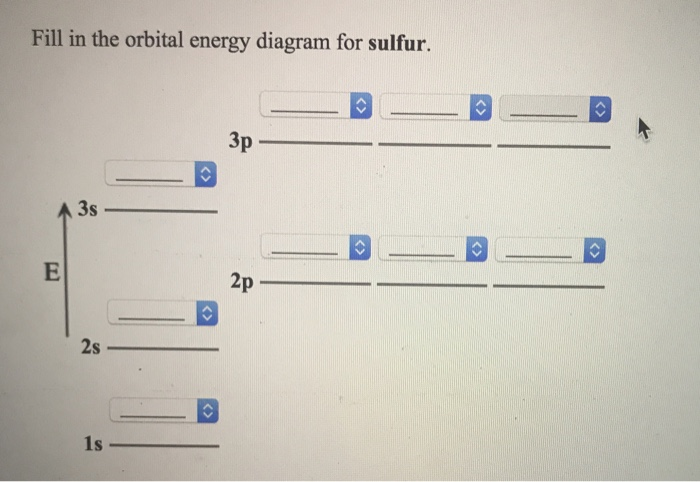
Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.

Orbital filling diagram for sulfur
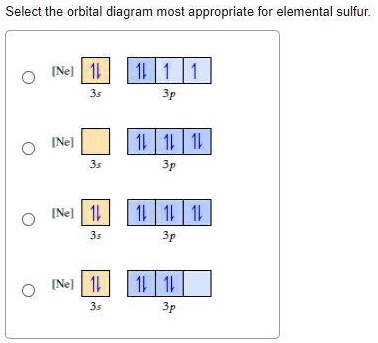
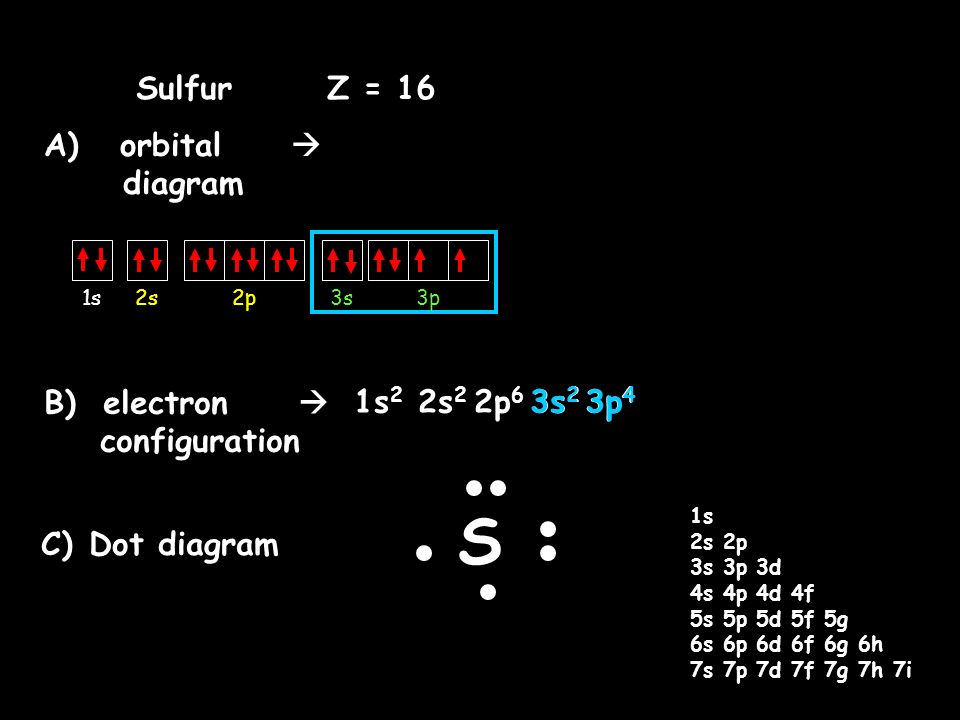
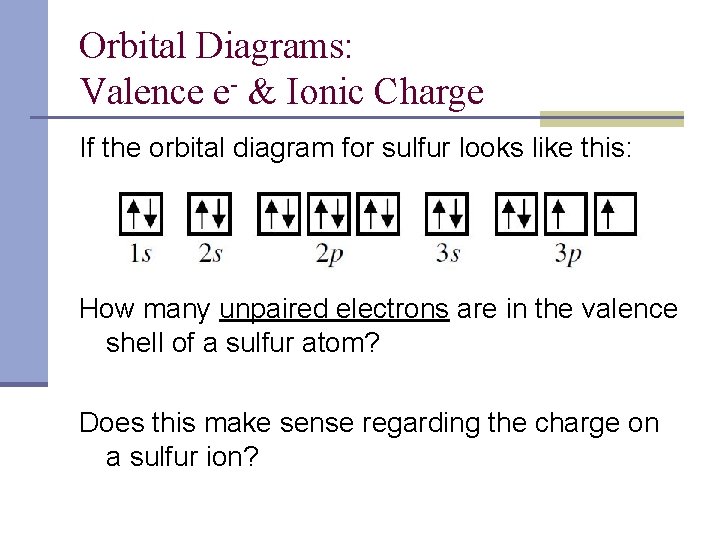
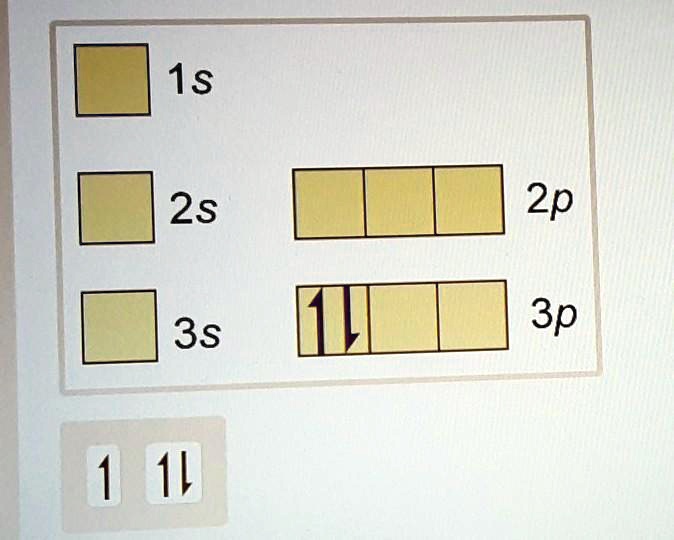
What Is the Orbital Diagram for Sulfur? - Reference.com What Is the Orbital Diagram for Sulfur? By Staff Writer Last Updated April 03, 2020 The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. Orbital Filling Diagram For Sulfur - schematron.org The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
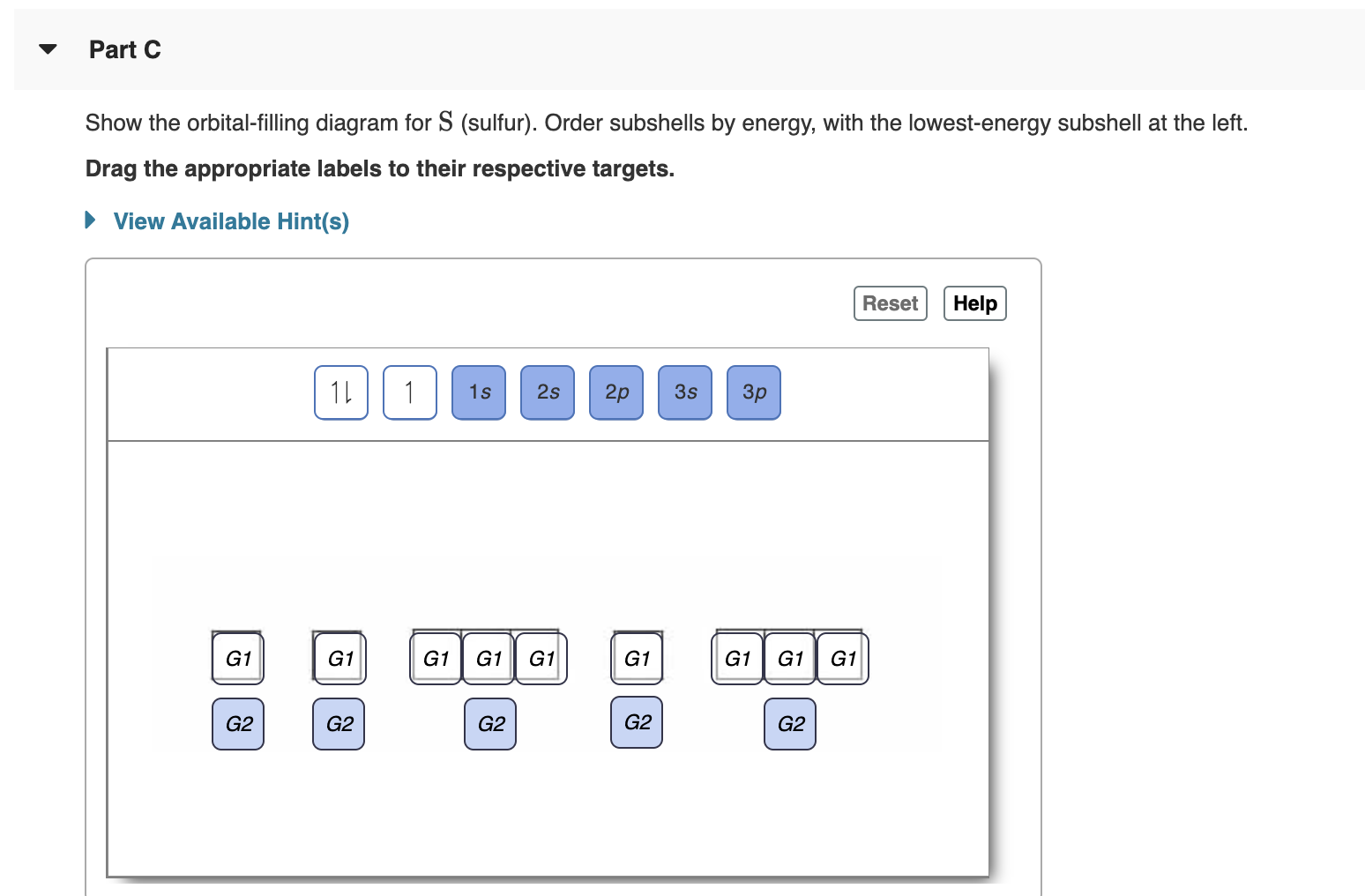
Orbital filling diagram for sulfur. Answered: Show the orbital-filling diagram for S… | bartleby Answered: Show the orbital-filling diagram for S… | bartleby. Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. Show The Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Electron Configurations - Department of Chemistry ... Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the … Show the orbital-filling diagram for sulfur. | Study.com Show the orbital-filling diagram for sulfur. Orbital configuration: In an atom, electrons are arranged around the nucleus in fixed shells. These electrons are arranged in increasing order of...
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... gemmaron.nl Mar 16, 2022 · 101.3 hampton roads. WHPE Translator. 1s orbital wave function (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ... Orbital Box Diagram For Sulfur - schematron.org Well, we use the aufbau principle, and for sulfur, Z= The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. boxes or lines represent each orbital. • arrows within boxes Draw an orbital diagram for beryllium (Z=4). 1s.
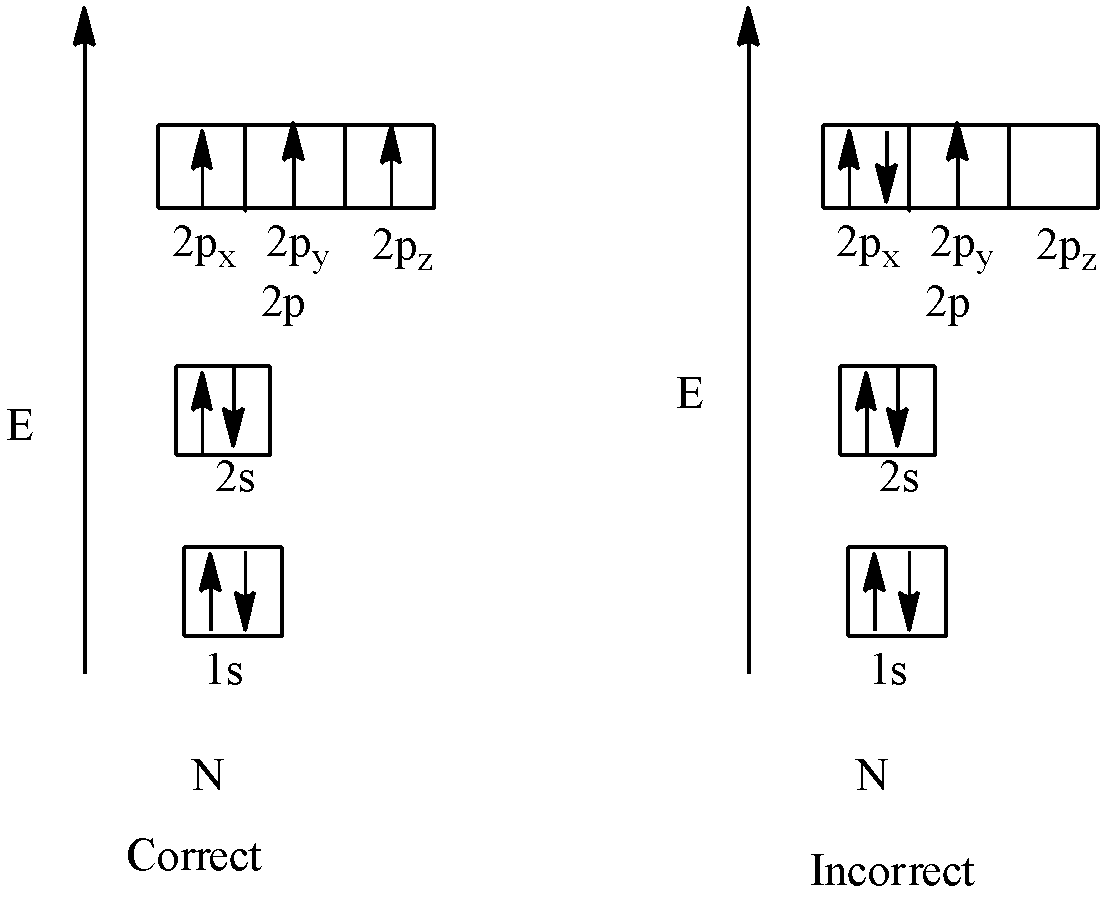
Chem Ch 3, Chem Ch 2 Flashcards - Quizlet An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. Lower energy subshells fill before higher energy subshells. The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Hints 3s Question: Show the orbital-filling diagram for S (sulfur). Show The Orbital Filling Diagram For Sulfur Each arrow represents one electron.Aug 04, · The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
OneClass: Part C Show the orbital-filling diagram for S ... Part C Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals.
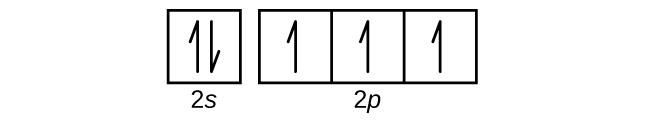
Electron Config & Orbital Filling Answer Key 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
techiescientist.com › c2h2-lewis-structureC2H2 Lewis Structure, Molecular Geometry, Hybridization ... 2 days ago · C2H2 Molecular Orbital (MO) Diagram. The Molecular Orbital (MO) Diagram is a pictorial representation of bonding taking place between the electrons of the participating atoms to produce new molecules. The basic principle this diagram follows is the atomic orbitals combine and overlaps in a certain manner to produce a similar number of molecular ...
Uncovering electrocatalytic conversion mechanisms from ... 1. Introduction. Lithium-sulfur (Li–S) battery is considered as a promising candidate for alternating traditional lithium-ion batteries, due to its ultra-high energy density, and the low-cost, environmental friendliness nature of sulfur , , .During discharge process, the active S 8 molecules are reduced to various lithium polysulfides (LiPSs) and finally Li 2 S through a 16-electron …
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: ... Electron configuration of elements Hund's rule and Orbital filling diagrams. Categories Uncategorized Post navigation. Germanium (Ge) - Periodic Table (Element ...
Show The Orbital-filling Diagram For Br (bromine) Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for . Show the orbital-filling diagram for Br (bromine).
Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 1 15 2s 2p 3s 3p G1 G1 G1 G1G1 G1 G1 G1 | G1 G2 G2 G2 G2 G2 Submit Part D Show the orbital-filling diagram for Br (bromine).
Electron Configuration Questions and Answers - Study.com Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the …
40 orbital diagram for sulfur - Wiring Diagrams Manual Show The Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals.
Electron configuration for Molybdenum (element 42 ... The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 4 But in reality, one electron moves from the 5s orbital to the 4d orbital: Electronic configuration of the Molybdenum atom in ascending order of orbital energies: 1s 2 ...
Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Show the orbital-filling diagram for Br (bromine).
opentextbc.ca › 8-4-molecular-orbital-theory8.4 Molecular Orbital Theory – Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Solved A Review Constants Periodic Table Learning ... - Chegg An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. Part C Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left.
Sulfur(S) electron configuration and orbital diagram Orbital diagram for sulfur (S) Sulfur (S) excited state electron configuration Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The valency of the element is determined by electron configuration in the excited state.
Chemistry 2.12: Electron Orbitals Flashcards | Quizlet What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 2. What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram. 10. Sulfur has an atomic number of 16. What is the electron configuration for an electronically neutral atom of sulfur?
PDF Work on Elctron configuration and orbital diagram 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
Chemistry Atomic concepts Flashcards | Quizlet Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for Br (bromine).
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Electron Configuration for Sulfur (S) - UMD In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Periodic Trends Flashcards - Quizlet Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p⁶3s²3p⁴
(PDF) Inorganic Chemistry 4th edition ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham
Electron Configuration & Orbital Filling Diagram Ws Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital . Place the ending configuration and the dot diagram for the above ...
H2S Lewis Structure, Molecular Geometry, Hybridization ... Mar 16, 2022 · H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals.
Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
Orbital Filling Diagram For Sulfur - schematron.org The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
What Is the Orbital Diagram for Sulfur? - Reference.com What Is the Orbital Diagram for Sulfur? By Staff Writer Last Updated April 03, 2020 The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins.


![Track UI] Unknown](https://user-images.githubusercontent.com/5923094/149025583-de00e2f6-f514-40b5-b29e-31575c887f4b.png)
















0 Response to "40 orbital filling diagram for sulfur"
Post a Comment